4.1 Therapeutic indications
Indicated in adults for the treatment of
- Chronic idiopathic constipation (CIC)
- Irritable bowel syndrome with constipation (IBS-C)
4.2 Posology and method of administration
The recommended dosage of Plecasoft for the treatment of CIC and IBS-C is 3 mg taken orally once daily. Preparation and administration instructions
- Plecasoft can be taken with or without food.
- If a dose is missed, skip the missed dose and take the next dose at the regular time. Do not take two doses at the same time.
- Swallow a tablet whole for each dose.
- For adult patients with swallowing difficulties, Plecasoft tablets can be crushed and administered orally either in applesauce or with water or administered with water via a nasogastric or gastric feeding tube. Mixing Plecasoft crushed tablets in other soft foods or in other liquids has not been tested.
Oral administration in applesauce
- In a clean container, crush the Plecasoft tablet to a powder and mix with 1 teaspoonful of room temperature applesauce.
- Consume the entire tablet-applesauce mixture immediately. Do not store the mixture for later use.
Oral administration in water
- Place the Plecasoft tablet in a clean cup.
- Pour approximately 30 mL of room temperature water into the cup.
- Mix by gently swirling the tablet and water mixture for at least 10 seconds. The Plecasoft tablet will fall apart in the water.
- Swallow the entire contents of the tablet water mixture immediately.
- If any portion of the tablet is left in the cup, add another 30 mL of water to the cup, swirl for at least 10 seconds, and swallow immediately.
- Do not store the tablet-water mixture for later use.
Administration with water via a nasogastric or gastric feeding tube
- Place the Plecasoft tablet in a clean cup with 30 mL of room temperature water.
- Mix by gently swirling the tablet and water mixture for at least 15 seconds. The Plecasoft tablet will fall apart in the water.
- Flush the nasogastric or gastric feeding tube with 30 mL of water using a catheter tip syringe.
- Draw up the mixture using the syringe and immediately administer via the nasogastric or gastric feeding tube. Do not reserve for future use.
- If any portion of the tablet is left in the cup, add another 30 mL of water to the cup, swirl for at least 15 seconds, and using the same syringe, administer via the nasogastric or gastric feeding tube.
- Using the same or a fresh syringe, flush the nasogastric or gastric feeding tube with at least 10 mL of water.
4.3 Contraindications
- Patients less than 6 years of age due to the risk of serious dehydration.
- Patients with known or suspected mechanical gastrointestinal obstruction.
4.4 Special warnings and precautions for use
Risk of serious dehydration in pediatric patients
Plecanatide is contraindicated in patients less than 6 years of age. The safety and effectiveness of Plecanatide in patients less than 18 years of age have not been established Due to increased intestinal expression of GC-C, patients less than 6 years of age may be more likely than patients 6 years of age and older to develop severe diarrhea and its potentially serious consequences. Avoid the use of Plecanatide in patients 6 years to less than 18 years of age. Diarrhea If severe diarrhea occurs, suspend dosing and rehydrate the patient.
4.5 Drugs interactions
Neither plecanatide nor its active metabolite inhibited the cytochrome P450 (CYP) enzymes 2C9 and 3A4, and they did not induce CYP3A4 in vitro. Plecanatide and its active metabolite were neither substrates nor inhibitors of the transporters Pglycoprotein (P-gp) or breast cancer resistance protein (BCRP) in vitro.
4.6 Use in special populations (such as pregnant women, lactating women, paediatric patients, geriatric patients etc.)
Pregnancy
Plecanatide and its active metabolite are negligibly absorbed systemically following oral administration and maternal use is not expected to result in fetal exposure to the drug. The available data on Plecanatide use in pregnant women are not sufficient to inform any drug associated risks for major birth defects and miscarriage. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes.
Lactation
There is no information regarding the presence of plecanatide in human milk, or its effects on milk production or the breastfed infant. Plecanatide and its active metabolite are negligibly absorbed systemically following oral administration. It is unknown whether the negligible systemic absorption of plecanatide by adults will result in a clinically relevant exposure to breastfed infants. Exposure to plecanatide in breastfed infants has the potential for serious adverse effects. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Plecanatide and any potential adverse effects on the breastfed infant from Plecanatide or from the underlying maternal condition.
Pediatric use
Plecanatide is contraindicated in pediatric patients less than 6 years of age. Avoid use of Plecanatide in patients 6 years to less than 18 years of age. The safety and effectiveness of Plecanatide in patients less than 18 years of age have not been established. Because of increased intestinal expression of GC-C, patients less than 6 years of age may be more likely than patients 6 years of age and older to develop diarrhea and its potentially serious consequences. Plecanatide is contraindicated in patients less than 6 years of age.
Geriatric use
The safety and effectiveness of Plecanatide in patients greater than 65 years of age have not been established.
4.7 Effects on ability to drive and use machines
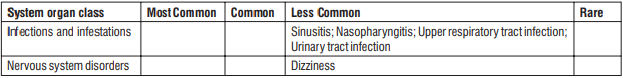
Plecanatide has no or negligible influence on the ability to drive and use machines. During treatment with Plecanatide, dizziness has been reported as less common adverse reaction. Therefore, patients who experience dizziness should be cautious while driving or using machines.
4.8 Undesirable effects

Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com Website : https://www.zuventus.co.in/drug-safety-reporting By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
There is no specific treatment to the event of overdose. In the event of overdose, the patient should be treated symptomatically and supportive measures instituted as required.


