BRAINMAX
Therapy Area
Neurodevelopmental
1.0 Generic name
OMEGA-3 LIQUID
2.0 Qualitative and quantitative composition
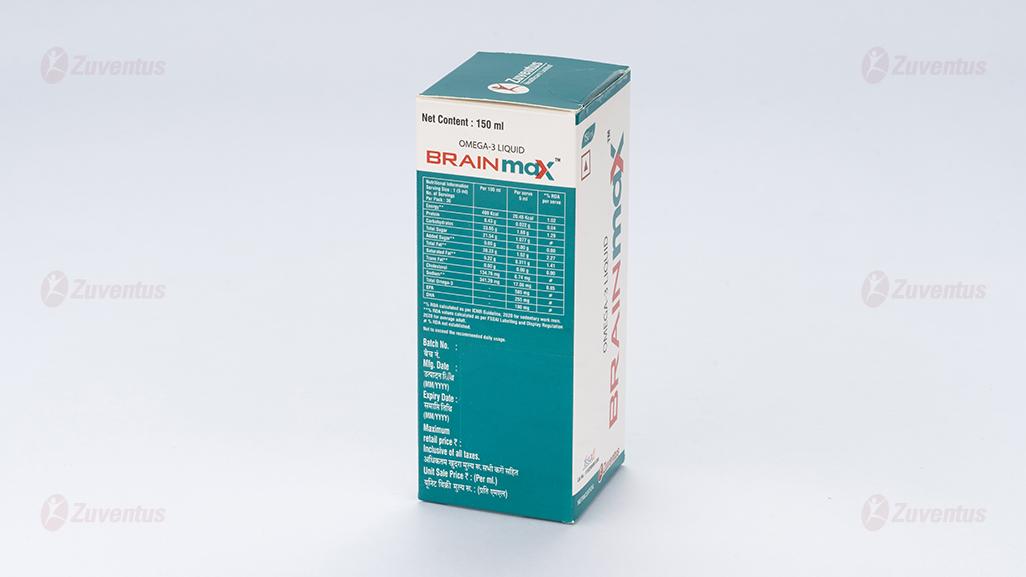
Nutritional Information:
Each Serving size of 5 ml contains:
- Total Omega-3………… 585 mg
- EPA ……………………. 255 mg
- DHA …………………….180mg
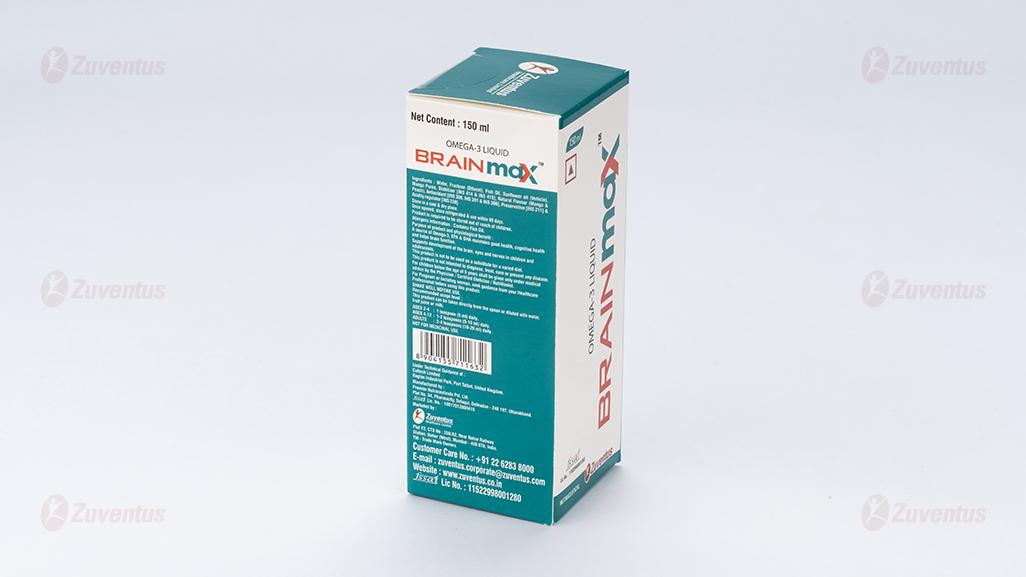
Ingredients: Water, Fructose, Fish Oil, Sunflower Oil, Mango Puree, Stabilizer [INS 414 & INS 415], Flavour - Mango & Peach, Antioxidant [INS 300, INS 301 & INS 307b], Preservative [INS 211] & Acidity regulator [INS 330].
3.0 Dosage form and strength
Dosage Form: liquid
Dosage Strength: 585 mg of omega−3 fatty acids per 5ml.
4.0 Clinical particulars
4.1 Therapeutic indication
Usage: BRAINMAXTM is a source of Omega-3 fatty acids, EPA & DHA, maintains good health, cognitive health and helps brain function. Supports development of the brain, eyes and nerves in children and adolescents.
4.2 Posology and method of administration
This product can be taken directly from the spoon or diluted with water, fruit juice or milk.
AGES 2-4 years: 1 teaspoon (5 ml) daily.
AGES 4-12 years: 1-2 teaspoons (5-10 ml) daily.
ADULTS: 2-4 teaspoons (10-20 ml) daily.
SHAKE WELL BEFORE USE.
4.3 Contraindications
BRAINMAX TM is contraindicated in patients known to be allergic to fish oil and ingredient of it.
4.4 Special warnings and precautions for use
- Allergen information: contains fish oil. BRAINMAXTM contain omega-3 fatty acids (EPA and DHA) obtained from the oil of several fish sources. It is not known whether patients with allergies to fish and/or shellfish, are at increased risk of an allergic reaction to BRAINMAXTM. BRAINMAXTM should be used with caution in patients with known hypersensitivity to fish and/or shellfish.
- It’s a Nutraceutical. NOT FOR MEDICINAL USE. This product is not intended to diagnose, treat, cure or prevent any disease.
- Not to exceed the stated recommended daily usage. Once opened, store refrigerated & use within 90 days.
- Please consult your healthcare provider, if you are taking blood thinners or are diabetic.
4.5 Drug interactions
Anticoagulants or Other Drugs Affecting Coagulation
Because of the moderate increase in bleeding time (with the high dosage), patients receiving anticoagulant therapy must be monitored and the dosage of anticoagulant adjusted if necessary.
4.6 Use in special populations
For Pregnant or lactating woman, seek guidance from your Healthcare Professional before using this product.
4.7 Effects on ability to drive and use machines
Effects on ability to drive and use machines have not been studied. Nevertheless, BRAINMAXTM is expected to have no or negligible influence on the ability to drive and use machines.
4.8 Undesirable effects
BRAINMAXTM has no, or minor side effects. When used in recommended doses, no side effects have been reported with BRAINMAXTM.
However, fish oil supplements can cause mild side effects, including:
- A fishy aftertaste, bad breath
- Heartburn, nausea or diarrhea
- Rash
- BRAINMAXTM does contain fish. Therefore, people allergic to fish will show allergic reaction to BRAINMAXTM.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to:medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
There are no special recommendations. Treatment should be symptomatic.
5.0 Pharmacological properties
5.1 Mechanism of Action/Pharmacodynamic properties
BRAINMAXTM contains essential fatty acids, omega-3 series polyunsaturated fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) extracted from fish oil. They are essential to health and people must consume them through food. Omega-3 fatty acids are considered as a functional food because of its functional component mainly polyunsaturated fatty acids, which has positive impact on human health when consumed in a sufficient quantity.
Omega-3 fatty acids are essential nutrients that play crucial roles in human health, including in pediatric patients. Here are some key points regarding their use in pediatric populations:
1. Brain Development: Omega-3 fatty acids, especially DHA, are crucial for brain development in infants and young children. DHA supports neuronal growth, synaptogenesis, and cognitive function.
2. Visual Development: DHA is also essential for the development of the retina and visual acuity in infants, contributing to healthy vision.
3. Behavioural Disorders: Some studies suggest that omega-3 supplementation may help in managing symptoms of ADHD (Attention-Deficit/Hyperactivity Disorder) and other behavioural disorders by supporting cognitive function and reducing inflammation.
4. Cardiovascular Health: Omega-3 fatty acids can contribute to cardiovascular health by lowering triglyceride levels and potentially reducing the risk of cardiovascular diseases later in life.
5.3 Pharmacokinetic properties
During and after absorption, there are three main pathways for the metabolism of the omega-3 fatty acids:
- the fatty acids are first transported to the liver where they are incorporated into various categories of lipoproteins and then channeled to the peripheral lipid stores;
- the cell membrane phospholipids are replaced by lipoprotein phospholipids and the fatty acids can then act as precursors for various eicosanoids;
- the majority is oxidised to meet energy requirements.
The concentration of omega-3 fatty acids, EPA and DHA, in the plasma phospholipids corresponds to the EPA and DHA incorporated into the cell membranes.
Animal pharmacokinetic studies have shown that there is a complete hydrolysis of the ethyl ester accompanied by satisfactory absorption and incorporation of EPA and DHA into the plasma phospholipids and cholesterol esters.
6.0 Nonclinical properties
6.1 Animal Toxicology
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, of repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction and development. In addition, non-clinical literature data on safety pharmacology are indicating that there is no hazard for humans.
7.0 Description
Nutritional Information:
Each Serving size of 5 ml contains:
- Total Omega-3………… 585 mg
- EPA ……………………. 255 mg
- DHA …………………….180mg
Ingredients: Water, Fructose, Fish Oil, Sunflower Oil, Mango Puree, Stabilizer [INS 414 & INS 415], Flavour - Mango & Peach, Antioxidant [INS 300, INS 301 & INS 307b], Preservative [INS 211] & Acidity regulator [INS 330].
8.0 Pharmaceutical particulars
8.1 Incompatibilities
None applicable
8.2 Shelf-life
Best before 24 months from manufacturing date.
8.3 Packaging information
Net Quantity: 150 ml
8.4 Storage and handing instructions
Store in a cool & dry place.
Once opened, store refrigerated & use within 90 days.
Keep out of reach of children.
9.0 Patient counselling information
- BRAINMAXTM should be used with caution in patients with known sensitivity or allergy to fish and/or shellfish
- If you are pregnant, nursing, taking any medications or have any medical condition, consult your doctor before use.
- Discontinue use and consult your doctor if any adverse reaction occurs.
- Please consult your healthcare provider, if you are taking blood thinners or are diabetic.
12.0 Date of revision
9th July 2024
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
- What BRAINMAXTM are and what it is used for
- What you need to know before you take BRAINMAXTM
- How to take BRAINMAXTM
- Possible side effects
- How to store BRAINMAXTM
- Contents of the pack and other information
1. What BRAINMAX is and what it is used for
BRAINMAXTM contains essential fatty acids, omega-3 series polyunsaturated fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) extracted from fish oil. They are essential to health and people must consume them through food. Omega-3 fatty acids are considered as a functional food because of its functional component mainly polyunsaturated fatty acids, which has positive impact on human health when consumed in a sufficient quantity.
Omega-3 fatty acids are essential nutrients that play crucial roles in human health, including in pediatric patients. Here are some key points regarding their use in pediatric populations:
1. Brain Development: Omega-3 fatty acids, especially DHA, are crucial for brain development in infants and young children. DHA supports neuronal growth, synaptogenesis, and cognitive function.
2. Visual Development: DHA is also essential for the development of the retina and visual acuity in infants, contributing to healthy vision.
3. Behavioural Disorders: Some studies suggest that omega-3 supplementation may help in managing symptoms of ADHD (Attention-Deficit/Hyperactivity Disorder) and other behavioural disorders by supporting cognitive function and reducing inflammation.
4. Cardiovascular Health: Omega-3 fatty acids can contribute to cardiovascular health by lowering triglyceride levels and potentially reducing the risk of cardiovascular diseases later in life.
Usage: BRAINMAXTM is a source of Omega-3 fatty acids, EPA & DHA, maintains good health, cognitive health and helps brain function. Supports development of the brain, eyes and nerves in children and adolescents.
2. What you need to know before you take BRAINMAX
Do not take BRAINMAXTM
- if patients are known to be allergic to fish oil (omega-3 fatty acids) and ingredient of it.
Warnings and precautions
Talk to your doctor, before taking BRAINMAXTM
- if you are due to have or have had surgery recently
- if you have had a trauma recently
- if you have a kidney problem
- if you have diabetes which is not controlled
- if you have/have had problems with your liver. Your doctor will monitor any effects BRAINMAXTM may have on your liver with blood tests.
Other medicines and BRAINMAXTM
Anticoagulants or Other Drugs Affecting Coagulation
Because of the moderate increase in bleeding time (with the high dosage), patients receiving anticoagulant therapy must be monitored and the dosage of anticoagulant adjusted if necessary.
Pregnancy, breast-feeding and fertility
For Pregnant or lactating woman, seek guidance from your Healthcare Professional before using this product.
Driving and using machines
BRAINMAXTM is expected to have no or negligible influence on the ability to drive and use machines.
3. How to take BRAINMAX
BrainmaxTM product can be taken directly from the spoon or diluted with water, fruit juice or milk.
Ages 2-4 years: 1 teaspoon (5 ml) daily.
Ages 4-12 years: 1-2 teaspoons (5-10 ml) daily.
Adults: 2-4 teaspoons (10-20 ml) daily.
Shake well before use.
Instructions for use
If you take more BRAINMAXTM than you should
If you accidentally take more of this medicine than you should, do not worry, as this is unlikely to need special treatment.
If you forget to take BRAINMAXTM
Do not take a double dose to make up for a forgotten dose. If you miss a dose, take it when you remember unless it is almost time for your next dose, in which case take the next dose as usual.
If you stop using BRAINMAXTM
Do not stop the treatment without consulting your doctor. Talk to your doctor if you are not satisfied with the effects of BRAINMAXTM. If you have any further questions on the use of this medicine, ask your doctor, pharmacist.
4. Possible side effects
BRAINMAXTM has no, or minor side effects. When used in recommended doses, no side effects have been reported with BRAINMAXTM.
However, fish oil supplements can cause mild side effects, including:
- A fishy aftertaste, bad breath
- Heartburn, nausea or diarrhea
- Rash
- BRAINMAXTM does contain fish. Therefore, people allergic to fish will show allergic reaction to BRAINMAXTM.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top of the home page.
https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store BRAINMAX
Store in a cool & dry place.
Once opened, store refrigerated & use within 90 days.
Keep out of reach of children.
6. Contents of the pack and other information
What BRAINMAXTM contain
Nutritional Information:
Each Serving size of 5 ml contains:
- Total Omega-3………… 585 mg
- EPA ……………………. 255 mg
- DHA …………………….180mg
Ingredients: Water, Fructose, Fish Oil, Sunflower Oil, Mango Puree, Stabilizer [INS 414 & INS 415], Flavour - Mango & Peach, Antioxidant [INS 300, INS 301 & INS 307b], Preservative [INS 211] & Acidity regulator [INS 330].
Marketing Authorisation Holder:
Zuventus Healthcare Limited
Plot Y2, CTS No: 358/A2, Near
Nahur Railway Station, Nahur
(West), Mumbai - 400 078, India.
TM - Trade Mark Owners.
Manufacturer:
Cultech Limited
Unit 2-3, Christchurch Road, Baglan Industrial Park,
Port Talbot, Neath Port Talbot, SA12 7BZ UK,
United Kingdom.
This leaflet was last revised in 08/2024