ICL 2 Injection
Therapy Area
Anti Infective
1.0 Generic Name
Colistimethate Sodium for Injection IP 1/2/3 Million IU
2.0 Qualitative and Quantitative Composition
ICL-1 Each vial contains:
Colistimethate Sodium IP 1,000,000 IU
(IU: International Units) Excipients q.s
ICL-2 Each vial contains:
Colistimethate Sodium IP 2,000,000 IU
(IU: International Units) Excipients q.s
ICL-3 Each vial contains:
Colistimethate Sodium IP 3,000,000 IU
(IU: International Units) Excipients q.s
3.0 Dosage Form and Strength
Sterile lyophilized powder for solution for injection and infusion; 1/2/3 Million IU
4.0 Clinical Particulars
4.1 Therapeutic indication
For the treatment of some serious infections caused by Gram-negative bacteria, including those of the lower respiratory tract and urinary tract, when more commonly used systemic antibacterial agents may be contraindicated or may be ineffective because of bacterial resistance.
4.2 Posology and method of administration
Intravenous.
The following dose recommendations are made based on limited population-pharmacokinetic data in critically ill patients:
Adults and adolescents
Maintenance dose 9 million IU/day in 2-3 divided doses.
In patients who are critically ill, a loading dose of 9 MIU should be administered.
The most appropriate time interval to the first maintenance dose has not been established.
Modelling suggests that loading and maintenance doses of up to 12 MIU may be required in patients with good renal function in some cases. Clinical experience with such doses is however extremely limited, and safety has not been established.
The loading dose applies to patients with normal and impaired renal functions including those on renal replacement therapy.
Renal impairment
Dose adjustments in renal impairment are necessary, but pharmacokinetic data available for patients with impaired renal function is very limited.
The following dose adjustments are suggested as guidance.
Dose reductions are recommended for patients with creatinine clearance < 50 ml/min:
Twice daily dosing is recommended.
|
Creatinine clearance (ml/min) |
Daily dose |
|
< 50- 30 |
5.5- 7.5 MIU |
|
<30- 10 |
4.5- 5.5 MIU |
|
<10 |
3.5 MIU |
MIU = million IU
Haemodialysis and continuous haemo(dia)filtration
Colistin appears to be dialyzable through conventional haemodialysis and continuous venovenous haemo(dia)filtration (CVVHF, CVVHDF). There are extremely limited data from population PK studies from very small numbers of patients on renal replacement therapy. Firm dose recommendations cannot be made. The following regimes could be considered.
Haemodialysis
- No-HD days: 2.25 MIU/day (2.2-2.3 MIU/day).
- HD days: 3 MIU/day on haemodialysis days, to be given after the HD session.
Twice daily dosing is recommended.
CVVHF/ CVVHDF
As in patients with normal renal function. Three times daily dosing is recommended.
Hepatic impairment
There are no data in patients with hepatic impairment. Caution is advised when administering colistimethate sodium in these patients.
Elderly
No dose adjustments in older patients with normal renal function are considered necessary.
Paediatric population
The data supporting the dose regimen in paediatric patients are very limited. Renal maturity should be taken into consideration when selecting the dose. The dose should be based on lean body weight.
Children ≤ 40kg
75,000-150,000 IU/kg/day divided into 3 doses.
For children with a body weight above 40 kg, use of the dosing recommendation for adults should be considered.
The use of doses >150,000 IU/kg/day has been reported in children with cystic fibrosis.
There are no data regarding the use or magnitude of a loading dose in critically ill children.
No dose recommendations have been established in children with impaired renal function.
Intrathecal and intraventricular administration
Based on limited data, the following dose is recommended in adults:
Intraventricular route
125,000 IU/day
Intrathecally administered doses should not exceed those recommended for intraventricular use.
No specific dosing recommendation can be made in children for intrathecal and intraventricular routes of administration.
Method of administration
Colomycin is administered intravenously as a slow infusion over 30 – 60 minutes.
Patients with a totally implantable venous access device (TIVAD) in place may tolerate a bolus injection of up to 2 million units in 10ml given over a minimum of 5 minutes.
Colistimethate sodium undergoes hydrolysis to the active substance colistin in aqueous solution. For dose preparation, particularly where combination of multiple vials is needed, reconstitution of the required dose must be performed using strict aseptic technique.
4.3 Contraindications
Hypersensitivity to the active substance, colistin or to polymyxin B
4.4 Special warnings and precautions for use
Consideration should be given to co-administering intravenous colistimethate sodium with another antibacterial agent whenever this is possible, taking into account the remaining susceptibilities of the pathogen(s) under treatment. As the development of resistance to intravenous colistin has been reported in particular when it is used as a monotherapy, co- administration with other antibacterial should also be considered in order to prevent the emergence of resistance.
There are limited clinical data on the efficacy and safety of intravenous colistimethate sodium. The recommended doses in all subpopulations are equally based on limited data (clinical and pharmacokinetic/ pharmacodynamics data). In particular, there are limited safety data for the use of high doses (> 6MIU/day) and the use of a loading dose, and for special populations (patients with renal impairment and the paediatric population). Colistimethate sodium should only be used when other, more commonly prescribed antibiotics are not effective or not appropriate.
Renal function monitoring should be performed at the start of treatment and regularly during treatment in all patients. The dose of colistimethate sodium should be adjusted according to creatinine clearance. Patients who are hypovolaemic or those receiving other potentially nephrotoxic drugs are at increased risk of nephrotoxicity from colistin. Nephrotoxicity has been reported to be associated with cumulative dose and treatment duration in some studies. The benefit of prolonged treatment duration should be balanced against the potentially increased risk of renal toxicity.
Few cases of pseudo-Bartter syndrome have been reported in children and adults with the intravenous use of colistimethate sodium. Monitoring of serum electrolytes should be started in suspected cases and appropriate management should be implemented, however, normalisation of electrolyte imbalance might not be achieved without discontinuation of colistimethate sodium.
Caution is advised when administering colistimethate sodium to infants < 1 year of age as renal function is not fully mature in this age group. Further, the effect of immature renal and metabolic function on the conversion of colistimethate sodium to colistin is not known.
In case of an allergic reaction, treatment with colistimethate sodium must be discontinued and appropriate measures implemented.
High serum concentrations of colistimethate sodium, which may be associated with overdosage or failure to reduce the dosage in patients with renal impairment, have been reported to lead to neurotoxic effects such as facial paraesthesia, muscle weakness, vertigo, slurred speech, vasomotor instability, visual disturbances, confusion, psychosis and apnoea. Monitoring should be performed for perioral paraesthesia and paraesthesia in the extremities, which are signs of overdose.
Colistimethate sodium is known to reduce the presynaptic release of acetyl-choline at the neuro-muscular junction and should be used in patients with myasthenia gravis with the greatest caution and only if clearly needed.
Respiratory arrest has been reported following intramuscular administration of colistimethate sodium. Impaired renal function increases the possibility of apnoea and neuromuscular blockade following administration of colistimethate sodium.
Colistimethate sodium should be used with extreme caution in patients with porphyria.
Antibiotic-associated colitis and pseudomembranous colitis have been reported with nearly all anti-bacterial agents and may occur with colistimethate sodium. They may range from mild to life-threatening in severity. It is important to consider this diagnosis in patients who develop diarrhoea during or after the use of colistimethate sodium. Discontinuation of therapy and the administration of specific treatment for Clostridium difficile should be considered. Medicinal products that inhibit peristalsis should not be given.
Intravenous colistimethate sodium does not cross the blood brain barrier to a clinically relevant extent. The use of intrathecal or intraventricular administration of colistimethate sodium in the treatment of meningitis was not systematically investigated in clinical trials and is supported by case reports only. Data supporting the posology are very limited. The most commonly observed adverse effect of CMS administration was aseptic meningitis.
4.5 Drugs interactions
Concomitant use of intravenous colistimethate sodium with other medications that are potentially nephrotoxic or neurotoxic should be undertaken with great caution.
Caution should be taken with concomitant use with other formulations of colistimethate sodium as there is little experience and there is a possibility of summative toxicity.
No in vivo interaction studies have been performed. The mechanism of conversion of colistimethate sodium to the active substance, colistin, is not characterised. The mechanism of colistin clearance, including renal handling, is equally unknown. Colistimethate sodium or colistin did not induce the activity of any P 450 (CYP) enzyme tested (CYP1A2, 2B6, 2C8, 2C9, 2C19 and 3A4/5) in in vitro studies in human hepatocytes.
The potential for drug-drug interactions should be borne in mind when colistimethate sodium is co-administered with drugs known to inhibit or induce drug metabolising enzymes or drugs known to be substrates for renal carrier mechanisms.
Due to the effects of colistin on the release of acetylcholine, non-depolarising muscle relaxants should be used with caution in patients receiving colistimethate sodium as their effects could be prolonged.
Co-treatment with colistimethate sodium and macrolides such as azithromycin and clarithromycin, or fluoroquinolones such as norfloxacin and ciprofloxacin should be undertaken with caution in patients with myasthenia gravis.
Concomitant use of colistimethate sodium with other medicinal products of neurotoxic and/or nephrotoxic potential should be avoided. These include the aminoglycoside antibiotics such as gentamicin, amikacin, netilmicin and tobramycin. There may be an increased risk of nephrotoxicity if given concomitantly with cephalosporin antibiotics.
4.6 Use in special populations
There are no adequate data from the use of colistimethate sodium in pregnant women. Single dose studies in human pregnancy show that colistimethate sodium crosses the placental barrier and there may be a risk of foetal toxicity if repeated doses are given to pregnant patients. Animal studies are insufficient with respect to the effect of colistimethate sodium on reproduction and development. Colistimethate sodium should be used in pregnancy only if the benefit to the mother outweighs the potential risk to the fetus.
Colistimethate sodium is secreted in breast milk. Colistimethate sodium should be administered to breastfeeding women only when clearly needed.
4.7 Effects on ability to drive and use machines
During parenteral treatment with Colistimethate Sodium neurotoxicity may occur with the possibility of dizziness, confusion or visual disturbance. Patients should be warned not to drive or operate machinery if these effects occur.
4.8 Undesirable effects
Systemic treatment
The likelihood of adverse events may be related to the age, renal function and condition of the patient.
- In cystic fibrosis patients neurological events have been reported in up to 27% of patients. These are generally mild and resolve during or shortly after treatment.
- Neurotoxicity may be associated with overdose, failure to reduce the dose in patients with renal insufficiency and concomitant use of either neuromuscular blocking drugs or other drugs with similar neurological effects. Reducing the dose may alleviate symptoms. Effects may include apnea, transient sensory disturbances (such as facial paraesthesia and vertigo) and, rarely, vasomotor instability, slurred speech, visual disturbances, confusion or psychosis.
- Adverse effects on renal function have been reported, usually following use of higher than recommended doses in patients with normal renal function, or failure to reduce the dosage in patients with renal impairment or during concomitant use of other nephrotoxic drugs. The effects are usually reversible on discontinuation of therapy.
- In cystic fibrosis patients treated within the recommended dosage limits, nephrotoxicity appears to be rare (less than 1%). In seriously ill hospitalised non-CF patients, signs of nephrotoxicity have been reported in approximately 20% of patients.
- Hypersensitivity reactions including skin rash and drug fever have been reported. If these occur treatment should be withdrawn.
- Local irritation at the site of injection may occur.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: http://www.zuventus.co.in/safety.aspx
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Overdose can result in neuromuscular blockade that can lead to muscular weakness, apnoea and possible respiratory arrest. Overdose can also cause acute renal failure characterised by decreased urine output and increased serum concentrations of BUN and creatinine.
There is no specific antidote, manage by supportive treatment. Measures to increase the rate of elimination of colistin e.g. mannitol diuresis, prolonged haemodialysis or peritoneal dialysis may be tried, but effectiveness is unknown.
5.0 Pharmacological Properties
5.1 Pharmacodynamic properties
Resistance
Resistant bacteria are characterised by modification of the phosphate groups of lipopolysaccharide, which become substituted with ethanolamine or aminoarabinose. Naturally resistant Gram-negative bacteria, such as Proteus mirabilis and Burkholderia cepacia, show complete substitution of their lipid phosphate by ethanolamine or aminoarabinose.
Cross resistance between colistin (polymyxin E) and polymyxin B is expected. Since the mechanism of action of the polymyxins is different from that of other antibacterial agents, resistance to colistin and polymyxin by the above mechanism alone would not be expected to result in resistance to other drug classes.
PK/PD relationship
Polymyxins have been reported to have a concentration-dependent bactericidal effect on susceptible bacteria. fAUC/ MIC is considered to be correlated with clinical efficacy.
|
EUCAST Breakpoints |
Susceptible (S) |
Resistant (R)a |
|
Acinetobacter |
S≤ 2 |
R>2 mg/L |
|
Enterobacteriaceae |
S≤ 2 |
R>2 mg/L |
|
Pseudomonas spp |
S≤ 4 |
R>4 mg/L |
a Breakpoints apply to dosage of 2-3 MIU x 3. A loading dose (9 MIU) may be needed.
Susceptibility
The prevalence of acquired resistance may vary geographically and with time for selected species and local information on resistance is desirable, particularly when treating severe infections. As necessary, expert advice should be sought when the local prevalence of resistance is such that the utility of the agent in at least some types of infections is questionable.
| Commonly susceptible species |
| Acinetobacter baumannii Haemophilus influenzae Klebsiella spp Pseudomonas aeruginosa |
| Species for which acquired resistance may be a problem |
| Stenotrophomonas maltophilia Achromobacter xylosoxidans (formerly Alcaligenes xylosoxidans) |
| Inherently resistant organisms |
| Burkholderia cepacia and related species. Proteus species Providencia species Serratia species |
5.2 Mechanism of Action
Colistin is a cyclic polypeptide antibacterial agent belonging to the polymyxin group. Polymyxins work by damaging the cell membrane and the resulting physiological effects are lethal to the bacterium. Polymyxins are selective for aerobic Gram-negative bacteria that have a hydrophobic outer membrane.
5.3 Pharmacokinetic properties
Absorption
The information on the pharmacokinetics of colistimethate sodium (CMS) and colistin is limited. There are indications that pharmacokinetics in critically ill patients differ from those in patients with less severe physiological derangement and from those in healthy volunteers. The following data are based on studies using HPLC to determine CMS/colistin plasma concentrations.
After infusion of colistimethate sodium the inactive pro-drug is converted to the active colistin. Peak plasma concentrations of colistin have been shown to occur with a delay of up to 7 hours after administration of colistimethate sodium in critically ill patients.
Absorption from the gastrointestinal tract does not occur to any appreciable extent in the normal individual.
Distribution
The volume of distribution of colistin in healthy subjects is low and corresponds approximately to extracellular fluid (ECF). The volume of distribution is relevantly enlarged in critically ill subjects. Protein binding is moderate and decreases at higher concentrations. In the absence of meningeal inflammation, penetration into the cerebrospinal fluid (CSF) is minimal, but increases in the presence of meningeal inflammation.
Both CMS and colistin display linear PK in the clinically relevant dose range.
Elimination
It is estimated that approximately 30% of colistimethate sodium is converted to colistin in healthy subjects, its clearance is dependent on creatinine clearance and as renal function decreases, a greater portion of CMS is converted to colistin. In patients with very poor renal function (creatinine clearance <30 ml/min), the extent of conversion could be as high as 60 to 70%. CMS is eliminated predominantly by the kidneys via glomerular filtration. In healthy subjects, 60% to 70% of CMS is excreted unchanged in the urine within 24 hours.
The elimination of the active colistin is incompletely characterised. Colistin undergoes extensive renal tubular reabsorption and may either be cleared non-renally or undergo renal metabolism with the potential for renal accumulation. Colistin clearance is decreased in renal impairment, possibly due to increased conversion of CMS.
Half-life of colistin in healthy subjects and those with cystic fibrosis is reported to be around 3h and 4h, respectively, with a total clearance of around 3L/h. In critically ill patients, half-life has been reported to be prolonged to around 9-18h.
6.0 Nonclinical Properties
6.1 Animal Toxicology or Pharmacology
Data on potential genotoxicity are limited and carcinogenicity data for colistimethate sodium are lacking. Colistimethate sodium has been shown to induce chromosomal aberrations in human lymphocytes, in vitro. This effect may be related to a reduction in mitotic index, which was also observed.
Reproductive toxicity studies in rats and mice do not indicate teratogenic properties. However, colistimethate sodium given intramuscularly during organogenesis to rabbits at 4.15 and 9.3 mg/kg resulted in talipes varus in 2.6 and 2.9% of fetuses respectively. These doses are 0.5 and 1.2 times the maximum daily human dose. In addition, increased resorption occurred at 9.3 mg/kg.
7.0 Description
Colistimethate Sodium is a polypeptide antibiotic.
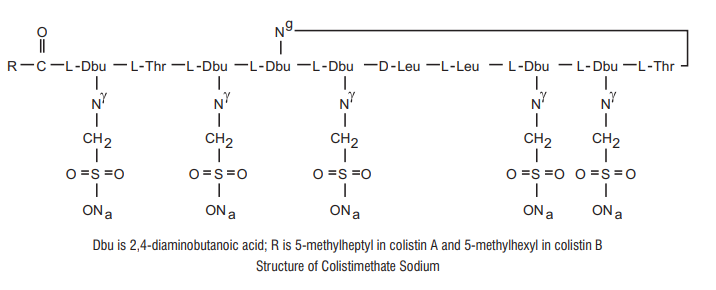
Chemical Name: N-(4-amino-1-(1-(4-amino-1-oxo-1-(3,12,23-tris(2-aminoethyl) -20-(1-hydroxyethyl)-6,9-diisobutyl-2,5,8,11,14,19,22-heptaoxo-1,4,7,10,13,18 -hexaazacyclotricosan-15-ylamino) butan-2-ylamino)-3-hydroxybutan-2 ylamino) -1-oxobutan-2-yl)-N,5-dimethylheptanamide.
Molecular Formula: C58H105N16 Na5028S5
Molecular Weight: 1750 kDa
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Mixed infusions, injections involving Colistimethate Sodium should be avoided. Addition of other antibiotics such as erythromycin, tetracycline and cephalothin to solution of Colistimethate Sodium may lead to precipitation
8.2 Shelf-life
Refer on pack
8.3 Packaging information
ICL-1: A vial of 1 million IU.
ICL-2: A vial of 2 million IU.
ICL-3: A vial of 3 million IU.
8.4 Storage and handing instructions
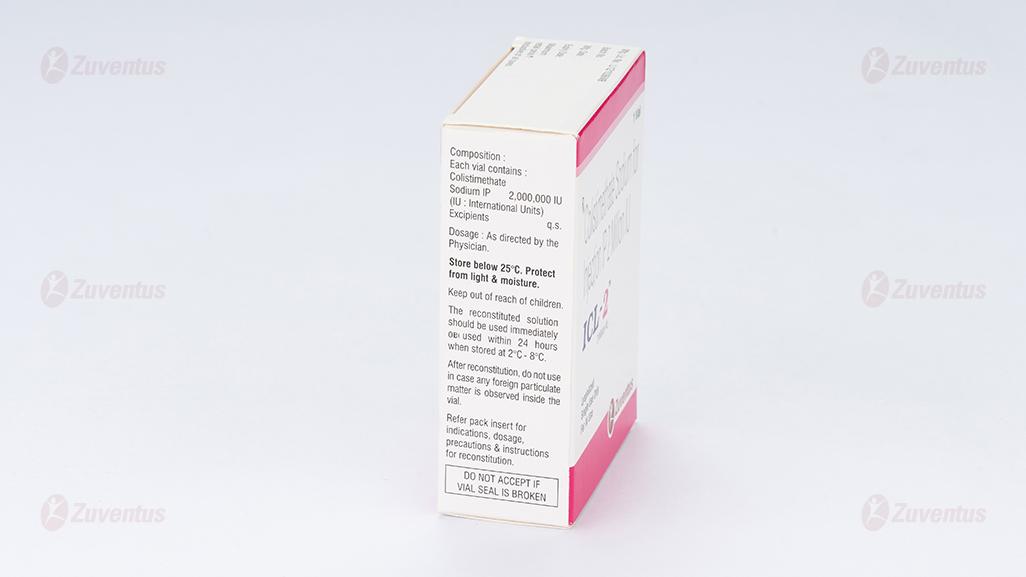
Store below 25°C. Protect from light & moisture. Keep out of reach of children. After reconstitution, do not use in case any foreign particulate matter is observed inside the vial. Reconstituted Solutions for Infusion or Injection The reconstituted solution should be used immediately or used within 24 hours when stored at 2°C -8°C.
9.0 Patient Counselling Information
Patients should be counseled that antibacterial drugs including Colistimethate for Injection, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Colistimethate for Injection, is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Colistimethate for Injection, or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
12. Date of revision
06/2024
About leaflet
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor, pharmacist or nurse.
This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What ICL injection is and what it is used for
2. What you need to know before you use ICL injection
3. How to use ICL injection
4. Possible side effects
5. How to store ICL injection
6. Contents of the pack and other information
1. What ICL injection is and what it is used for
ICL contains the active substance colistimethate sodium. Colistimethate sodium is an antibiotic. It belongs to a group of antibiotics that are called polymyxins.
ICL is given by injection to treat some types of serious infections caused by certain bacteria. ICL is used when other antibiotics are not suitable.
2. What you need to know before you use ICL injection
Do not use ICL
If you are allergic to colistimethate sodium, colistin or to other polymyxins.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using ICL
- If you have or have had kidney problems.
- If you suffer from myasthenia gravis
- If you suffer from porphyria
- If you suffer from asthma
Children
In premature and new-born babies, special care should be taken when using ICL as the kidneys are not yet fully developed
Other medicines and ICL
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
If you are taking any of the following medicines, you may or may not be able to take ICL. Sometimes the other medicines must be stopped (if only for a while) or you may need a lower dose of ICL or you may need to be monitored while you are taking ICL. In some cases, the level of ICL in your blood may have to be measured from time to time to make sure that you are having the right dose.
medicines like antibiotics called aminoglycosides (which include gentamicin, tobramycin, amikacin and netilmicin) and cephalosporins which can affect how your kidneys function. Taking such medicines at the same time as ICL can increase the risk of damage to the kidneys.
medicines like antibiotics called aminoglycosides (which include gentamicin, tobramycin, amikacin and netilmicin) which can affect your nervous system. Taking such medicines at the same time as ICL can increase the risk of side effects in the ears and other parts of your nervous system.
medicines called muscle relaxants, often used during general anaesthesia. ICL can increase the effects of these medicines. If you have a general anaesthetic, let your anaesthetist know that you are having ICL.
If you suffer from myasthenia gravis and are also taking other antibiotics called macrolides (such as azithromycin, clarithromycin or erythromycin) or antibiotics called fluoroquinolones (such as ofloxacin, norfloxacin and ciprofloxacin), taking ICL further increases the risk of muscle weakness and breathing difficulties.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, or think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. There is no information about the safety of ICL in pregnant women. Your doctor should advise you before using ICL where benefits of the medicine exceed the risk. Colistimethate sodium may be secreted in the breast milk. Please discuss the use of ICL with your doctor.
Driving and using machines
ICL may make you feel dizzy, confused or have problems with your sight, such as blurred vision. If this happens to you, do not drive or use any tools or machines.
3. How to take ICL injection
Depending on the reason (see section 1 of this leaflet), ICL may be given by fast injection (over 5 minutes into a special kind of tube in a vein) or slow injection (infusion over about 30 to 60 minutes) into a vein. ICL may occasionally be given by injection into the brain or the spine
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
For use by infusion or injection:
ICL is given to you by your doctor as an infusion into a vein over 30 - 60 minutes.
The usual daily dose in adults is 9 million units, divided into two or three doses. If you are quite unwell, you will be given a higher dose of 9 million units once at the start of treatment
In some cases, your doctor may decide to give a higher daily dose of up to 12 million units.
The usual daily dose in children weighing up to 40 kg is 75,000 to 1,50,000 units per kilogram body weight, divided into three doses. Higher doses have occasionally been given in cystic fibrosis.
Children and adults with kidney problems, including those on dialysis, are usually given lower doses.
Your doctor will monitor your kidney function regularly while you receive ICL.
Method of administration:
ICL is given by injection mainly in hospitals. If you are to treat yourself at home, your doctor, pharmacist or nurse will show you how to dissolve the powder and inject the right dose of solution.
Duration of treatment:
Your doctor will decide how long your treatment should last, depending on the severity of the infection. When treating bacterial infections, it is important to complete the full course of treatment so as to prevent worsening of the existing infection.
If you use more ICL than you should
If you think that you have given yourself too much ICL, you should contact your doctor or nurse Immediately for advice or, if they are not available, contact or go to your nearest hospital accident and emergency department. If too much ICL is accidentally given, the side effects can be serious and can include kidney problems, muscle weakness and difficulty (or even stopping) breathing.
If you are being treated in hospital or at home by a doctor or nurse and think that you may have missed a dose or been given too much ICL, please ask your doctor, nurse or pharmacist about this.
If you forget to use ICL
If you are treating yourself and have missed any doses, you should give the missed dose as soon as you remember and then give the next dose 8 hours later if using ICL three times a day, or 12 hours later if using ICL twice a day. Carry on from there as instructed. Do not take a double dose to make up for a forgotten dose.
If you stop using ICL
Do not stop your treatment early unless your doctor says you can. Your doctor will decide how long your treatment should last.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them
Allergic reactions
When ICL is given into a vein, an allergic reaction is possible. Serious allergic reactions can happen even with the very first dose and can include rapid development of rashes, swelling of the face, tongue and neck, inability to breathe due to narrowing of the airways and loss of consciousness.
If you experience signs of an allergic reaction you should seek urgent medical attention.
Less severe allergic reactions include skin rashes that appear later during treatment.
Side effects associated with injecting ICL into a vein
Side effects that affect the nervous system are more likely to occur when the dose of ICL is too high, in people who have poor kidneys or in those who are also taking muscle relaxants or other medicines with a similar effect on how the nerves work.
The most serious of these possible side effects in the nervous system is inability to breathe because of paralysis of the chest muscles. If you experience any difficulty breathing you should seek urgent medical attention.
Other possible side effects include numbness or tingling (especially around the face), dizziness or loss of balance, rapid changes in blood pressure or blood flow (including faintness and flushing), slurred speech, problems with vision, confusion and mental problems (including loss of sense of reality). There can be reactions at the site of the injection, such as irritation.
Kidney problems may also occur. These are especially likely in people who already have poor kidneys, or who are given ICL at the same time as other medicines that can cause side effects in the kidneys or who are given a dose that is too high. These problems will normally get better if treatment is stopped or the dose of ICL is reduced.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store ICL injection
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the vial label and the carton after EXP. The expiry date refers to the last day of that month.
Do not store above 25 0 C. Keep the vials in the outer carton in order to protect from light.
ICL solutions for injection and for inhalation should be used immediately after preparation.
If this is not possible, talk first to your doctor or pharmacist as the solutions may be stored in a refrigerator for no longer than 24 hours. Any remaining solution should be discarded.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What ICL injection contains
The active substance is Colistimethate Sodium.
Colistimethate Sodium for Injection IP 1 Million IU
Composition:
Each vial contains:
Colistimethate Sodium IP…1,000,000 IU (IU: International Units)
Excipients q.s.
Colistimethate Sodium for Injection IP 2 Million IU
Composition:
Each vial contains:
Colistimethate Sodium IP 2,000,000 IU (IU: International Units)
Excipients q.s.
Colistimethate Sodium for Injection IP 3 Million IU
Composition:
Each vial contains:
Colistimethate Sodium IP 3,000,000 IU (IU: International Units)
Excipients q.s.
Pack size/ presentation:
1 Vial