Ivadin XR Tablets
Therapy Area
Cardiology
1.0 Generic name
vabradine Prolonged Release Tablets 10 mg
2.0 Qualitative and quantitative composition
Each film coated prolonged release
tablet contains :
Ivabradine Hydrochloride
equivalent to Ivabradine 10 mg
Excipients q.s
Colours : Red Oxide of Iron Lake
Yellow Oxide of Iron Lake & Titanium Dioxide IP
3.0 Dosage form and strength
Prolonged release tablet, 10 mg
4.0 Clinical particulars
4.1 Therapeutic indications
For the treatment of chronic stable angina pectoris in patients with coronary artery disease and treatment of chronic heart failure
4.2 Posology and method of administration Symptomatic treatment of chronic stable angina pectoris
It is recommended that the decision to initiate or titrate treatment takes place with the availability of serial heart rate measurements, ECG or ambulatory 24-hour monitoring. The starting dose of Ivadin XR 10 mg should be one tablet once a day, in patients aged below 75 years. After three to four weeks of treatment, if the patient is still symptomatic, if the initial dose is well tolerated and if resting heart rate remains above 60 bpm, the dose may be increased to the next higher dose in patients receiving 10 mg per day. The maintenance dose should not exceed 15 mg per day. If there is no improvement in symptoms of angina within 3 months after start of treatment, treatment of Ivabradine should be discontinued. In addition, discontinuation of treatment should be considered if there is only limited symptomatic response and when there is no clinically relevant reduction in resting heart rate within three months. If, during treatment, heart rate decreases below 50 beats per minute (bpm) at rest or the patient experiences symptoms related to bradycardia such as dizziness, fatigue or hypotension, the dose must be titrated downward including the lowest dose of 2.5 mg twice daily (one half 5 mg tablet twice daily). After dose reduction, heart rate should be monitored. Treatment must be discontinued if heart rate remains below 50 bpm or symptoms of bradycardia persist despite dose reduction.
Treatment of chronic heart failure
The treatment has to be initiated only in patient with stable heart failure. It is recommended that the treating physician should be experienced in the management of chronic heart failure. The usual recommended starting dose of Ivadin XR 10 mg should be one tablet once a day. After two weeks of treatment, the dose can be increased to 7.5 mg twice daily if resting heart rate is persistently above 60 bpm or decreased to 2.5 mg twice daily (one half 5 mg tablet twice daily) if resting heart rate is persistently below 50 bpm or in case of symptoms related to bradycardia such as dizziness, fatigue or hypotension. If heart rate is between 50 and 60 bpm, the dose of 10 mg once a day should be maintained. If during treatment, heart rate decreases persistently below 50 beats per minute (bpm) at rest or the patient experiences symptoms related to bradycardia, the dose must be titrated downward to the next lower dose in patients receiving 10 mg per day. If heart rate increases persistently above 60 beats per minute at rest, the dose can be up titrated to the next upper dose in patients receiving 10 mg per day. Treatment must be discontinued if heart rate remains below 50 bpm or symptoms of bradycardia persist
Special population
Elderly
In patients aged 75 years or more, a lower starting dose should be considered for these patients (2.5 mg twice daily) before up-titration if necessary
Renal impairment
No dose adjustment is required in patients with renal insufficiency and creatinine clearance above 15 ml/min.
No data are available in patients with creatinine clearance below 15 ml/min. Ivabradine should therefore be used with precaution in this population.
Hepatic impairment
No dose adjustment is required in patients with mild hepatic impairment. Caution should be exercised when using Ivabradine in patients with moderate hepatic impairment. Ivabradine is contraindicated for use in patients with severe hepatic insufficiency, since it has not been studied in this population and a large increase in systemic exposure is anticipated.
Paediatric population
The safety and efficacy of Ivabradine for the treatment of chronic heart failure in children aged below 18 years have not been established.
Method of administration
Tablets must be taken orally
4.3 Contraindications
• Hypersensitivity to the active substance or to any of the excipients listed in the formulation.
• Resting heart rate below 70 beats per minute prior to treatment
• Cardiogenic shock
• Acute myocardial infarction
• Severe hypotension (< 90/50 mmHg)
• Severe hepatic insufficiency
• Sick sinus syndrome
• Sino-atrial block
• Unstable or acute heart failure
• Pacemaker dependent (heart rate imposed exclusively by the pacemaker)
• Unstable angina rd
• AV-block of 3 degree
• Combination with strong cytochrome P450 3A4 inhibitors such as azole antifungals (Ketoconazole, Itraconazole), macrolide antibiotics (Clarithromycin, Erythromycin per os, Josamycin, Telithromycin), HIV protease inhibitors (Nelfinavir, Ritonavir) and Nefazodone.
• Combination with Verapamil or Diltiazem which are moderate CYP3A4 inhibitors with heart rate reducing properties.
• Pregnancy, lactation and women of child-bearing potential not using appropriate contraceptive measures.
4.4 Special warnings and precautions for use
Special warnings
Lack of benefit on clinical outcomes in patients with symptomatic chronic stable angina pectoris
Ivabradine is indicated only for symptomatic treatment of chronic stable angina pectoris because Ivabradine has no benefits on cardiovascular outcomes (e.g. myocardial infarction or cardiovascular death).
Measurement of heart rate
Given that the heart rate may fluctuate considerably over time, serial heart rate measurements, ECG or ambulatory 24-hour monitoring should be considered when determining resting heart rate before initiation of Ivabradine treatment and in patients on treatment with Ivabradine when titration is considered. This also applies to patients with a low heart rate, in particular when heart rate decreases below 50 bpm, or after dose reduction.
Cardiac arrhythmias
vabradine is not effective in the treatment or prevention of cardiac arrhythmias and likely loses its efficacy when a tachyarrhythmia occurs (eg. ventricular or supraventricular tachycardia). Ivabradine is therefore not recommended in patients with atrial fibrillation or other cardiac arrhythmias that interfere with sinus node function. In patients treated with Ivabradine the risk of developing atrial fibrillation is increased. Atrial fibrillation has been more common in patients using concomitantly amiodarone or potent class I anti-arrhythmics. It is recommended to regularly clinically monitor Ivabradine treated patients for the occurrence of atrial fibrillation (sustained or paroxysmal), which should also include ECG monitoring if clinically indicated (e.g. in case of exacerbated angina, palpitations, irregular pulse). Patients should be informed of signs and symptoms of atrial fibrillation and be advised to contact their physician if these occur. If atrial fibrillation develops during treatment, the balance of benefits and risks of continued Ivabradine treatment should be carefully reconsidered.
Chronic heart failure patients with intraventricular conduction defects (bundle branch block left, bundle branch block right) and ventricular dyssynchrony should be monitored closely.
Use in patients with AV nd -block of 2 degree
vabradine is not recommended in patients with AV-block of 2 degree.
Use in patients with a low heart rate
Ivabradine must not be initiated in patients with a pre-treatment resting heart rate below 70 beats per minute. If, during treatment, resting heart rate decreases persistently below 50 bpm or the patient experiences symptoms related to bradycardia such as dizziness, fatigue or hypotension, the dose must be titrated downward or treatment discontinued if heart rate below 50 bpm or symptoms of bradycardia persist.
Combination with calcium channel blockers
Concomitant use of Ivabradine with heart rate reducing calcium channel blockers such as verapamil or diltiazem is contraindicated. No safety issue has been raised on the combination of Ivabradine with nitrates and dihydropyridine calcium channel blockers such as amlodipine. Additional efficacy of Ivabradine in combination with dihydropyridine calcium channel blockers has not been established.
Chronic heart failure
Heart failure must be stable before considering Ivabradine treatment. Ivabradine should be used with caution in heart failure patients with NYHA functional classification IV due to limited amount of data in this population.
Stroke
The use of Ivabradine is not recommended immediately after a stroke since no data is available in these situations.
Visual function
vabradine influences retinal function. There is no evidence of a toxic effect of long-term Ivabradine treatment on the retina. Cessation of treatment should be considered if any unexpected deterioration in visual function occurs. Caution should be exercised in patients with retinitis pigmentosa.
Precautions for use
Patients with hypotension
Limited data are available in patients with mild to moderate hypotension, and Ivabradine should therefore be used with caution in these patients. Ivabradine is contraindicated in patients with severe hypotension (blood pressure < 90/50 mmHg).
Atrial fibrillation - Cardiac arrhythmias
There is no evidence of risk of (excessive) bradycardia on return to sinus rhythm when pharmacological cardioversion is initiated in patients treated with Ivabradine. However, in the absence of extensive data, non urgent DC-cardioversion should be considered 24 hours after the last dose of Ivabradine.
Use in patients with congenital QT syndrome or treated with QT prolonging medicinal products
The use of Ivabradine in patients with congenital QT syndrome or treated with QT prolonging medicinal products should be avoided. If the combination appears necessary, close cardiac monitoring is needed. Heart rate reduction, as caused by Ivabradine, may exacerbate QT prolongation, which may give rise to severe arrhythmias, in particular Torsade de pointes
Hypertensive patients requiring blood pressure treatment modifications
When treatment modifications are made in chronic heart failure patients treated with Ivabradine blood pressure should be monitored at an appropriate interval.
4.5 Interaction with other medicinal products and other forms of interaction
Pharmacodynamic interactions
Concomitant use not recommended
QT prolonging medicinal products
- Cardiovascular QT prolonging medicinal products (e.g. quinidine, disopyramide, bepridil, sotalol, ibutilide, amiodarone).
- Non cardiovascular QT prolonging medicinal products (e.g. pimozide, ziprasidone, sertindole, mefloquine, halofantrine, pentamidine, cisapride, intravenous erythromycin).
The concomitant use of cardiovascular and non cardiovascular QT prolonging medicinal products with Ivabradine should be avoided since QT prolongation may be exacerbated by heart rate reduction. If the combination appears necessary, close cardiac monitoring is needed.
Concomitant use with precaution
Potassium-depleting diuretics (thiazide diuretics and loop diuretics) : hypokalaemia can increase the risk of arrhythmia. As Ivabradine may cause bradycardia, the resulting combination of hypokalaemia and bradycardia is a predisposing factor to the onset of severe arrhythmias, especially in patients with long QT syndrome, whether congenital or substance-induced. Pharmacokinetic interactions
Cytochrome P450 3A4 (CYP3A4)
vabradine is metabolised by CYP3A4 only and it is a very weak inhibitor of this cytochrome. Ivabradine was shown not to influence the metabolism and plasma concentrations of other CYP3A4 substrates (mild, moderate and strong inhibitors). CYP3A4 inhibitors and inducers are liable to interact with Ivabradine and influence its metabolism and pharmacokinetics to a clinically significant extent. Drug-drug interaction studies have established that CYP3A4 inhibitors increase Ivabradine plasma concentrations, while inducers decrease them. Increased plasma concentrations of Ivabradine may be associated with the risk of excessive bradycardia.
Contraindication of concomitant use
The concomitant use of potent CYP3A4 inhibitors such as azole antifungals (ketoconazole, itraconazole), macrolide antibiotics (Clarithromycin, Erythromycin per os, Josamycin, Telithromycin), HIV protease inhibitors (Nelfinavir, Ritonavir) and Nefazodone is contraindicated. The potent CYP3A4 inhibitors Ketoconazole (200 mg once daily) and Josamycin (1 g twice daily) increased Ivabradine mean plasma exposure by 7 to 8 fold. Moderate CYP3A4 inhibitors : specific interaction studies in healthy volunteers and patients have shown that the combination of Ivabradine with the heart rate reducing agents diltiazem or verapamil resulted in an increase in Ivabradine exposure (2 to 3 fold increase in AUC) and an additional heart rate reduction of 5 bpm. The concomitant use of Ivabradine with these medicinal products is contraindicated.
Concomitant use not recommended
Grapefruit juice : Ivabradine exposure was increased by 2-fold following the co-administration with grapefruit juice. Therefore the intake of grapefruit juice should be avoided.
Concomitant use with precautions
• Moderate CYP3A4 inhibitors : the concomitant use of Ivabradine with other moderate CYP3A4 inhibitors (e.g. Fluconazole) may be considered at the starting dose of 2.5 mg twice daily and if resting heart rate is above 70 bpm, with monitoring of heart rate.
• CYP3A4 inducers : CYP3A4 inducers (e.g. Rifampicin, Barbiturates, Phenytoin, Hypericumperforatum [St John's Wort]) may decrease Ivabradine exposure and activity. The concomitant use of CYP3A4 inducing medicinal products may require an adjustment of the dose of Ivabradine. The combination of Ivabradine 10 mg twice daily with St John's Wort was shown to reduce Ivabradine AUC by half. The intake of St John's Wort should be restricted during the treatment with Ivabradine.
Other concomitant use
Specific drug-drug interaction studies have shown no clinically significant effect of the following medicinal products on pharmacokinetics and pharmacodynamics of Ivabradine : proton pump inhibitors (Omeprazole, Lansoprazole), Sildenafil, HMG CoA reductase inhibitors (Simvastatin), dihydropyridine calcium channel blockers (Amlodipine, Lacidipine), Digoxin and Warfarin. In addition there was no clinically significant effect of Ivabradine on the pharmacokinetics of simvastatin, amlodipine, lacidipine, on the pharmacokinetics and pharmacodynamics of Digoxin, Warfarin and on the pharmacodynamics of Aspirin.
In pivotal phase III clinical trials the following medicinal products were routinely combined with Ivabradine with no evidence of safety concerns : angiotensin converting enzyme inhibitors, angiotensin II antagonists, beta-blockers, diuretics, anti-aldosterone agents, short and long acting nitrates, HMG CoA reductase inhibitors, fibrates, proton pump inhibitors, oral antidiabetics, aspirin and other anti-platelet medicinal products.
Paediatric population
Interaction studies have only been performed in adults.
4.6 Use in special populations
Women of child-bearing potential
Women of child-bearing potential should use appropriate contraceptive measures during treatment.
Pregnancy
There are no or limited amount of data from the use of Ivabradine in pregnant women. Studies in animals have shown reproductive toxicity. These studies have shown embryotoxic and teratogenic effects. The potential risk for humans is unknown. Therefore, Ivabradine is contraindicated during pregnancy
Breast-feeding
Animal studies indicate that Ivabradine is excreted in milk. Therefore, Ivabradine is contraindicated during breast-feeding. Women that need treatment with Ivabradine should stop breast-feeding, and choose for another way of feeding their child.
Fertility
Studies in rats have shown no effect on fertility in males and females.
4.7 Effects on ability to drive and use machines
A specific study to assess the possible influence of Ivabradine on driving performance has been performed in healthy volunteers where no alteration of the driving performance was evidenced. However, in post-marketing experience, cases of impaired driving ability due to visual symptoms have been reported. Ivabradine may cause transient luminous phenomena consisting mainly of phosphenes. The possible occurrence of such luminous phenomena should be taken into account when driving or using machines in situations where sudden variations in light intensity may occur, especially when driving at night.
vabradine has no influence on the ability to use machines.
4.8 UNDESIRABLE EFFECT
The most common adverse reactions with Ivabradine, luminous phenomena (phosphenes) and bradycardia, are dose dependent and related to the pharmacological effect of the medicinal product.
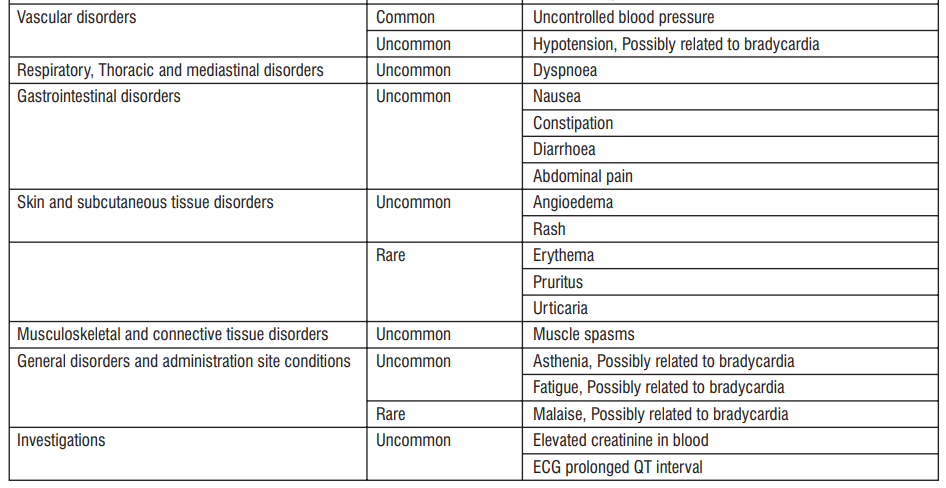
Tabulated list of adverse reactions
The following adverse reactions have been reported during clinical trials and are ranked using the following frequency : Very common (≥1/10); Common (≥1/100 to <1/10); Uncommon (≥1/1,000 to <1/100); Rare (≥1/10,000 to <1/1,000); Very rare (<1/10,000); Not known (cannot be estimated from the available data).

Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Symptoms
Overdose may lead to severe and prolonged bradycardia
Management
Severe bradycardia should be treated symptomatically in a specialised environment. In the event of bradycardia with poor haemodynamic tolerance, symptomatic treatment including intravenous beta-stimulating medicinal products such as isoprenaline may be considered. Temporary cardiac electrical pacing may be instituted if required.
5.0 Pharmacological properties
5.1 Mechanism of action
Ivabradine is a pure heart rate lowering agent, acting by selective and specific inhibition of the cardiac pacemaker I current that controls the f spontaneous diastolic depolarisation in the sinus node and regulates heart rate. The cardiac effects are specific to the sinus node with no effect on intra-atrial, atrioventricular or intraventricular conduction times, nor on myocardial contractility or ventricular repolarisation. Ivabradine can interact also with the retinal current Ihwhich closely resembles cardiac I . It participates in the temporal resolution of the visual system, f by curtailing the retinal response to bright light stimuli. Under triggering circumstances (e.g. rapid changes in luminosity), partial inhibition of Ih by Ivabradine underlies the luminous phenomena that may be occasionally experienced by patients. Luminous phenomena (phosphenes) are described as a transient enhanced brightness in a limited area of the visual field.
5.2 Pharmacodynamic properties
The main pharmacodynamic property of Ivabradine in humans is a specific dose dependent reduction in heart rate. Analysis of heart rate reduction with doses up to 20 mg twice daily indicates a trend towards a plateau effect which is consistent with a reduced risk of severe bradycardia below 40 bpm. At usual recommended doses, heart rate reduction is approximately 10 bpm at rest and during exercise. This leads to a reduction in cardiac workload and myocardial oxygen consumption. Ivabradine does not influence intracardiac conduction, contractility (no negative inotropic effect) or ventricular repolarisation :
• In clinical electrophysiology studies, Ivabradine had no effect on atrioventricular or intraventricular conduction times or cor rected QT intervals;
• In patients with left ventricular dysfunction (left ventricular ejection fraction (LVEF) between 30 and 45%), Ivabradine did not have any deleterious influence on LVEF.
5.3 Pharmacokinetic properties
Ivabradine is the S-enantiomer with no bioconversion demonstrated in vivo. The N-desmethylated derivative of Ivabradine has been identified as the main active metabolite in humans.
Absorption and bioavailability
Ivabradine is rapidly and almost completely absorbed after oral administration with a peak plasma level reached in about 1 hour under fasting condition. The absolute bioavailability of the film-coated tablets is around 40%, due to first-pass effect in the gut and liver. Food delayed absorption by approximately 1 hour, and increased plasma exposure by 20 to 30%. The intake of the tablet during meals is recommended in order to decrease intra-individual variability in exposure.
Distribution
vabradine is approximately 70% plasma protein bound and the volume of distribution at steady state is close to 100 l in patients. The maximum plasma concentration following chronic administration at the recommended dose of 5 mg twice daily is 22 ng/ml (CV=29%). The average plasma concentration is 10 ng/ml (CV=38%) at steady state
Biotransformation
vabradine is extensively metabolised by the liver and the gut by oxidation through cytochrome P450 3A4 (CYP3A4) only. The major active metabolite is the N-desmethylated derivative (S 18982) with an exposure about 40% of that of the parent compound. The metabolism of this active metabolite also involves CYP3A4. Ivabradine has low affinity for CYP3A4, shows no clinically relevant CYP3A4 induction or inhibition and is therefore unlikely to modify CYP3A4 substrate metabolism or plasma concentrations. Inversely, potent inhibitors and inducers may substantially affect Ivabradine plasma concentrations.
Elimination
vabradine is eliminated with a main half-life of 2 hours (70 - 75% of the AUC) in plasma and an effective half-life of 11 hours. The total clearance is about 400 ml/min and the renal clearance is about 70 ml/min. Excretion of metabolites occurs to a similar extent via faeces and urine. About 4% of an oral dose is excreted unchanged in urine.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential. Reproductive toxicity studies showed no effect of Ivabradine on fertility in male and female rats. When pregnant animals were treated during organogenesis at exposures close to therapeutic doses, there was a higher incidence of foetuses with cardiac defects in the rat and a small number of foetuses with ectrodactylia in the rabbit.
In dogs given Ivabradine (doses of 2, 7 or 24 mg/kg/day) for one year, reversible changes in retinal function were observed but were not associated with any damage to ocular structures. These data are consistent with the pharmacological effect of Ivabradine related to its interaction with hyperpolarisation-activated Ih currents in the retina, which share extensive homology with the cardiac pacemaker I current. f Other long-term repeat dose and carcinogenicity studies revealed no clinically relevant changes.
7.0 Description
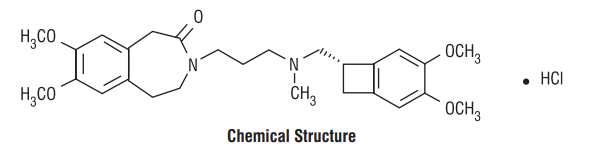
Ivabradine is a hyperpolarization-activated cyclic nucleotide-gated channel blocker that reduces the spontaneous pacemaker activity of the cardiac sinus node by selectively inhibiting the If current, resulting in heart rate reduction with no effect on ventricular repolarization and no effects on myocardial contractility. The chemical name for Ivabradine is 3-(3-{[((7S)-3,4-Dimethoxybicyclo[4.2.0] octa-1,3,5-trien-7-yl)methyl] methyl amino} propyl)-1,3,4,5-tetrahydro-7,8-dimethoxy-2H-3-benzazepin-2-one, hydrochloride. The molecular formula is C27H36N2O5• HCl and the molecular weight (free base + HCl) is 505.1 g/mol (468.6 + 36.5 g/mol).
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not known
8.2 Shelf-life
Please see manufacturing date and expiry date printed on pack. Do not use the product after the expiry date which is stated on the packaging. The expiry date refers to the last day of that month.
8.3 Packaging information
Alu-Alu blister strip of 10 tablets.
8.4 Storage and handing instructions
Store at a temperature not exceeding 30°C. Protect from moisture.
Keep out of reach of children.
9.0 Patient counselling information
• Fetal Toxicity Advise pregnant women of the potential risks to a fetus. Advise females of reproductive potential to use effective contraception and to notify their healthcare provider with a known or suspected pregnancy.
• Low Heart Rate Advise patients to report significant decreases in heart rate or symptoms such as dizziness, fatigue, or hypotension.
• Atrial Fibrillation Advise patients to report symptoms of atrial fibrillation, such as heart palpitations or racing, chest pressure, or worsened shortness of breath.
• Phosphenes Advise patients about the possible occurrence of luminous phenomena (phosphenes). Advise patients to use caution if they are driving or using machines in situations where sudden changes in light intensity may occur, especially when driving at night. Advise patients that phosphenes may subside spontaneously during continued treatment with Ivadin XR 10 mg.
• Drug Interactions Advise patients to avoid ingestion of grapefruit juice and St. John's wort.
• Intake with Food Advise patients to take Ivadin XR 10 mg once daily with food. Patients should be counselled on the appropriate use of Ivabradine with close monitoring on the dosages and side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again. If you have any further questions, ask your doctor, pharmacist or nurse. This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Ivadin-XR tablet is and what it is used for
2. What you need to know before you use Ivadin-XR tablet
3. How to use Ivadin-XR tablet
4. Possible side effects
5. How to store Ivadin-XR tablet
6. Contents of the pack and other information
1. What Ivadin-XR tablet is and what it is used for
Ivadin-XR (ivabradine) is a heart medicine used to treat:
Symptomatic stable angina pectoris (which causes chest pain) in adult patients whose heart rate is over or equal to 70 beats per minute. It is used in adult patients who do not tolerate or cannot take heart medicines called beta-blockers. It is also used in combination with beta-blockers in adult patients whose condition is not fully controlled with a beta-blocker.
Chronic heart failure in adult patients whose heart rate is over or equal to 75 beats per minute. It is used in combination with standard therapy, including beta-blocker therapy or when beta-blockers are contraindicated or not tolerated.
About stable angina pectoris (usually referred to as "angina"): Stable angina is a heart disease which happens when the heart does not receive enough oxygen. The most common symptom of angina is chest pain or discomfort.
About chronic heart failure: Chronic heart failure is a heart disease which happens when your heart cannot pump enough blood to the rest of your body. The most common symptoms of heart failure are breathlessness, fatigue, tiredness and ankle swelling.
2. What you need to know before you use Ivadin-XR tablet
Do not take Ivadin-XR
if you are allergic to ivabradine or any of the other ingredients of this medicine;
if your resting heart rate before treatment is too slow (below 70 beats per minute);
if you are suffering from cardiogenic shock (a heart condition treated in hospital);
if you suffer from a heart rhythm disorder (sick sinus syndrome, sino-atrial block, 3rd-degree AV block);
if you are having a heart attack;
if you suffer from very low blood pressure;
if you suffer from unstable angina (a severe form in which chest pain occurs very frequently and with or without exertion);
if you have heart failure which has recently become worse;
if your heartbeat is exclusively imposed by your pacemaker;
if you suffer from severe liver problems;
if you are already taking medicines for the treatment of fungal infections (such as ketoconazole, itraconazole), macrolide antibiotics (such as josamycin, clarithromycin, telithromycin or erythromycin given orally), medicines to treat HIV infections (such as nelfinavir, ritonavir), nefazodone (medicine to treat depression) or diltiazem, verapamil (used for high blood pressure or angina pectoris); if you are a woman able to have children and not using reliable contraception;
if you are pregnant or trying to become pregnant; if you are breast-feeding.
Warnings and precautions
Talk to your doctor or pharmacist before taking Ivadin-XR
if you suffer from heart rhythm disorders (such as irregular heartbeat, palpitation, increase in chest pain) or sustained atrial fibrillation (a type of irregular heartbeat), or an abnormality of electrocardiogram (ECG) called ‘long QT syndrome’;
if you have symptoms such as tiredness, dizziness or shortness of breath (this could mean that your heart is slowing down too much);
if you suffer from symptoms of atrial fibrillation (pulse rate at rest unusually high (over 110 beats per minute) or irregular, without any apparent reason, making it difficult to measure);
if you have had a recent stroke (cerebral attack);
if you suffer from mild to moderate low blood pressure;
if you suffer from uncontrolled blood pressure, especially after a change in your antihypertensive treatment;
if you suffer from severe heart failure or heart failure with abnormality of ECG called ‘bundle branch block;
if you suffer from chronic eye retinal disease;
if you suffer from moderate liver problems;
if you suffer from severe renal problems.
If any of the above applies to you, talk straight away to your doctor before or while taking Ivadin-XR.
Children
Do not give this medicine to children and adolescents younger than 18 years. Available data are insufficient in this age group.
Other medicines and Ivadin-XR tablet
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Make sure to tell your doctor if you are taking any of the following medicines, as a dose adjustment of Ivadin-XR or monitoring should be required:
fluconazole (an antifungal medicine);
rifampicin (an antibiotic);
barbiturates (for difficult sleeping or epilepsy);
phenytoin (for epilepsy);
Hypericum perforatum or St John’s Wort (herbal treatment for depression);
QT prolonging medicines to treat either heart rhythm disorders or other conditions:
- quinidine, disopyramide, ibutilide, sotalol, amiodarone (to treat heart rhythm disorders);
- bepridil (to treat angina pectoris);
- certain types of medicines to treat anxiety, schizophrenia or other psychoses (such as pimozide, ziprasidone, sertindole);
- anti-malarial medicines (such as mefloquine or halofantrine);
- intravenous erythromycin (an antibiotic); pentamidine (an antiparasitic medicine);
- cisapride (against the gastro-oesophageal reflux);
Some types of diuretics which may cause decrease in blood potassium level, such as furosemide, hydrochlorothiazide, indapamide (used to treat oedema, high blood pressure).
Ivadin-XR with food and drink
Avoid grapefruit juice during treatment with Ivadin-XR.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. Do not take Ivadin-XR if you are pregnant or are planning to have a baby (see "Do not take Ivadin-XR").
If you are pregnant and have taken Ivadin-XR, talk to your doctor.
Do not take Ivadin-XR if you are able to become pregnant unless you use reliable contraceptive measures (see "Do not take Ivadin-XR").
Do not take Ivadin-XR if you are breast-feeding (see "Do not take Ivadin-XR"). Talk to your doctor if you are breast-feeding or intending to breast-feed as breast-feeding should be discontinued if you take Ivadin-XR.
Driving and using machines
Ivadin-XR may cause temporary luminous visual phenomena (a temporary brightness in the field of vision, see “Possible side effects"). If this happens to you, be careful when driving or using machines at times when there could be sudden changes in light intensity, especially when driving at night.
3. How to take Ivadin-XR tablet
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Ivadin-XR should be taken during meals.
Symptomatic treatment of chronic stable angina pectoris
It is recommended that the decision to initiate or titrate treatment takes place with the availability of serial heart rate measurements, ECG or ambulatory 24-hour monitoring.
The starting dose of Ivadin-XR 10 mg should be one tablet once a day, in patients aged below 75 years. After three to four weeks of treatment, if the patient is still symptomatic, if the initial dose is well tolerated and if resting heart rate remains above 60 bpm, the dose may be increased to the next higher dose in patients receiving 10 mg per day. The maintenance dose should not exceed 15 mg per day.
If there is no improvement in symptoms of angina within 3 months after start of treatment, treatment of ivabradine should be discontinued.
In addition, discontinuation of treatment should be considered if there is only limited symptomatic response and when there is no clinically relevant reduction in resting heart rate within three months.
If, during treatment, heart rate decreases below 50 beats per minute (bpm) at rest or the patient experiences symptoms related to bradycardia such as dizziness, fatigue or hypotension, the dose must be titrated downward including the lowest dose of 2.5 mg twice daily (one half 5 mg tablet twice daily). After dose reduction, heart rate should be monitored. Treatment must be discontinued if heart rate remains below 50 bpm or symptoms of bradycardia persist despite dose reduction.
Treatment of chronic heart failure
The treatment has to be initiated only in patient with stable heart failure. It is recommended that the treating physician should be experienced in the management of chronic heart failure.
The usual recommended starting dose of Ivadin-XR 10 mg should be one tablet once a day. After two weeks of treatment, the dose can be increased to 7.5 mg twice daily if resting heart rate is persistently above 60 bpm or decreased to 2.5 mg twice daily (one half 5 mg tablet twice daily) if resting heart rate is persistently below 50 bpm or in case of symptoms related to bradycardia such as dizziness, fatigue or hypotension. If heart rate is between 50 and 60 bpm, the dose of 10 mg once a day should be maintained.
If during treatment, heart rate decreases persistently below 50 beats per minute (bpm) at rest or the patient experiences symptoms related to bradycardia, the dose must be titrated downward to the next lower dose in patients receiving 10 mg per day. If heart rate increases persistently above 60 beats per minute at rest, the dose can be up titrated to the next upper dose in patients receiving 10 mg per day.
Treatment must be discontinued if heart rate remains below 50 bpm or symptoms of bradycardia persist
Special population
Elderly
In patients aged 75 years or more, a lower starting dose should be considered for these patients (2.5 mg twice daily) before up-titration if necessary.
Renal impairment
No dose adjustment is required in patients with renal insufficiency and creatinine clearance above 15 ml/min.
No data are available in patients with creatinine clearance below 15 ml/min. Ivabradine should therefore be used with precaution in this population.
Hepatic impairment
No dose adjustment is required in patients with mild hepatic impairment. Caution should be exercised when using ivabradine in patients with moderate hepatic impairment. Ivabradine is contraindicated for use in patients with severe hepatic insufficiency, since it has not been studied in this population and a large increase in systemic exposure is anticipated.
If you take more Ivadin-XR than you should:
A large dose of Ivadin-XR could make you feel breathless or tired because your heart slows down too much. If this happens, contact your doctor immediately.
If you forget to take Ivadin-XR:
If you forget to take a dose of Ivadin-XR, take the next dose at the usual time. Do not take a double dose to make up for the forgotten dose.
The calendar printed on the blister containing the tablets should help you remember when you last took a tablet of Ivadin-XR.
If you stop taking Ivadin-XR:
As the treatment for angina or chronic heart failure is usually life-long, you should discuss with your doctor before stopping this medicinal product.
If you think that the effect of Ivadin-XR is too strong or too weak, talk to your doctor or pharmacist. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
If you use more Ivadin-XR tablet than you should
Tell your doctor immediately if you have taken more than the prescribed dose of this medicine. Take the medicine pack with you, even if there are no tablets left.
If you take more Ivadin-XR than recommended, you may have an increased risk of bleeding. If bleeding occurs, surgery, blood transfusions, or other treatments that may reverse anti-factor Xa activity may be required.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The most common adverse reactions with this medicine are dose dependent and related to its mode of action:
Very common (may affect more than 1 in 10 people)
Luminous visual phenomena (brief moments of increased brightness, most often caused by sudden changes in light intensity). They can also be described as a halo, coloured flashes, image decomposition or multiple images. They generally occur within the first two months of treatment after which they may occur repeatedly and resolve during or after treatment.
Common (may affect up to 1 in 10 people)
Modification in the heart functioning (the symptoms are a slowing down of the heart rate). They particularly occur within the first 2 to 3 months of treatment initiation.
Other side effects have also been reported:
Common (may affect up to 1 in 10 people)
Irregular rapid contraction of the heart (atrial fibrillation), abnormal perception of heartbeat (bradycardia, ventricular extrasystoles, 1st-degree AV block (ECG prolonged PQ interval)), uncontrolled blood pressure, headache, dizziness and blurred vision (cloudy vision).
Uncommon (may affect up to 1 in 100 people)
Palpitations and cardiac extra beats, feeling sick (nausea), constipation, diarrhoea, abdominal pain, spinning sensation (vertigo), difficulty breathing (dyspnoea), muscle spasms, high blood levels of uric acid, an excess of eosinophils (a type of white blood cell) and elevated creatinine in blood (a breakdown product of muscle), skin rash, angioedema (such as swollen face, tongue or throat, difficulty in breathing or swallowing), low blood pressure, fainting, feeling of tiredness, feeling of weakness, abnormal ECG heart tracing, double vision, impaired vision.
Rare (may affect up to 1 in 1,000 people)
Urticaria, itching, skin reddening, feeling unwell.
Very rare (may affect up to 1 in 10,000 people)
Irregular heartbeats (2nd-degree AV block, 3rd-degree AV block, sick sinus syndrome).
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top of the home page. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Ivadin-XR tablet
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister after ‘EXP’. The expiry date refers to the last day of that month.
This medicine does not require any special storage conditions.
Do not throw away any medicine via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What Ivadin-XR tablet contains
The active substance is ivabradine (as hydrochloride).
Composition:
Ivadin-XR 10 mg Tablets
Each film coated tablet contains:
Ivabradine (as hydrochloride) …10 mg
Excipients...q.s
Pack size/ presentation:
Ivadin-XR 10 mg tablets: 2 Tablets