Lornit Syrup
Therapy Area
Gastrointestinal
1.0 Generic name
L-Ornithine L-Aspartate syrup
2.0 Qualitative and quantitative composition
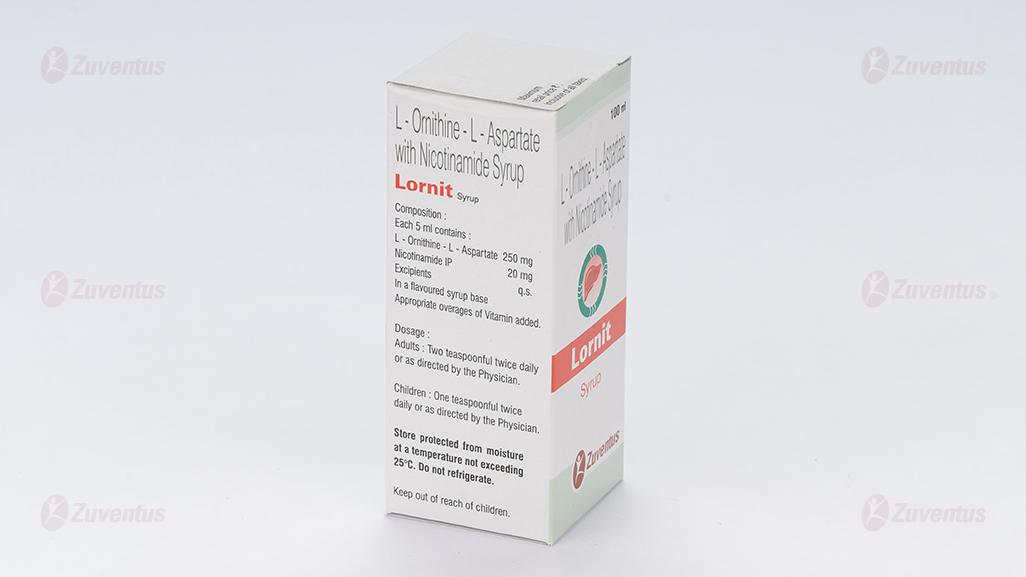
Each 5 mL contains:
L-Ornithine-L-Aspartate………………250 mg
Nicotinamide IP ……………………….20 mg
In a flavoured syrup base
Appropriate overages of Vitamins added
3.0 Dosage form and strength
Syrup
4.0 Clinical particulars
4.1 Therapeutic indication
Treatment of associated conditions and sequelae of diseases with impaired hepatic detoxification (e.g. cirrhosis of the liver), when there are symptoms and signs of minimal or overt hepatic encephalopathy.
4.2 Posology and method of administration
Adults: Two teaspoonful twice daily.
Children: One teaspoonful twice daily.
4.3 Contraindications
- Hypersensitivity to LOLA or any other excipients of this product.
- Severe renal insufficiency (serum creatinine value > 3 mg/100 ml).
4.4 Special warnings and precautions for use
- Monitoring of serum and urinary urea levels at regular intervals should be done.
- Should be used during pregnancy only if the potential benefits out-weigh the potential risk to the fetus.
4.5 Drugs interactions
No known drug-drug interaction.
4.6 Use in special populations
Pregnancy & Lactation
The administration in pregnancy and lactation should be avoided. If treatment is nevertheless
thought to be necessary, the benefits and risks should be carefully assessed.
4.7 Effects on ability to drive and use machines
No studies on the effect on the ability to drive and use machines have been performed.
4.8 Undesirable effects
Very rarely side effects like nausea and vomiting occur. These side effects are usually transient and do not necessitate the withdrawal of the drug.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to:medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
There is no data available for Lornit syrup overdosage. If any patient consumes excess of drug, it should be managed symptomatically.
5.0 Pharmacological properties
5.1 Mechanism of Action/ Pharmacodynamic properties
L-Ornithine-L-Aspartate is a stable salt of the amino acids ornithine and aspartic acid and provides substrates for urea genesis and glutamine synthesis, which are important mechanisms in ammonia detoxification. It is well known that both ornithine and aspartic acid play a key role in the liver metabolism. Ornithine brings ammonia into urea cycle, thereby converting ammonia into urea, a nontoxic substance. The other component of the drug, aspartic acid, not only takes part in an important stage in the reaction sequence involved in the urea cycle, but also features in the tricarboxylic acid cycle as oxaloacetate formed by transamination, thereby improving the energy balance of the diseased liver. Furthermore, aspartic acid promotes natural regeneration of the liver cells by taking part in pryimidine biosynthesis.
Nicotinamide (niacinamide), is the component of two coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) necessary for lipid metabolism, tissue respiration, glycogenolysis, inhibition of very low-density lipoprotein (VLDL) synthesis. It may increase chylomicron triglyceride removal from plasma.
5.3 Pharmacokinetic properties
- L-Ornithine-L-Aspartate is rapidly absorbed and cleaved into L-Ornithine and L-Aspartate.
- Elimination half-life of each amino acid is short, approximately 40 min and bioavailability is 82.2 28% after oral administration.
- Some L-Aspartate appears unchanged in the urine.
Following oral administration nicotinamide is rapidly absorbed (60-76%) with peak plasma time varying from 30-60 min. It mainly metabolizes in the liver with half-life of about 20-45 min. Nicotinamide is excreted in urine (60-88% as unchanged drug).
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
No known animal toxicology data
7.0 Description
Lornit Syrup is a supportive and maintenance therapy during mild to moderate liver-related concerns. It acts as a hepatic protector. It is used as a liver therapy.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
Refer on pack
9.0 Patient counselling information
- You will be regularly monitored for blood creatinine and blood/urine urea levels.
- Inform your doctor if you are pregnant, planning a pregnancy, or breastfeeding.
- Do not take this medicine if you are allergic to any of its ingredients.
12.0 Date of revision
23.09.2024
About Leaflet
Please read this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
- What Lornit syrup is and what it is used for
- What you need to know before you take Lornit syrup
- How to take Lornit syrup
- Possible side effects
- How to store Lornit syrup
- Contents of the pack and other information
1. What Lornit Syrup is and what it is used for
Lornit Syrup contains the active substances L-Ornithine L-Aspartate and Nicotinamide. It is used to treat conditions associated with impaired liver detoxification, such as cirrhosis of the liver, and symptoms of hepatic encephalopathy.
2. What you need to know before you take Lornit syrup
Do not take Lornit Syrup if:
- You are allergic to L-Ornithine L-Aspartate, Nicotinamide, or any of the other ingredients of this medicine.
- You have severe renal insufficiency (serum creatinine value > 3 mg/100 ml).
Warnings and precautions:
- Regular monitoring of serum and urinary urea levels is recommended.
- Use during pregnancy should only occur if the potential benefits outweigh the risks.
Other medicines and Lornit Syrup:
- No known drug interactions.
Pregnancy and breastfeeding:
- Avoid use during pregnancy and breastfeeding unless deemed necessary by your doctor.
Driving and using machines:
No studies have been conducted on the effects of this medicine on the ability to drive and use machines.
3. How to take Lornit syrup
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Adults:
- Two teaspoonfuls twice daily.
Children:
- One teaspoonful twice daily.
Shake the bottle well before use. You can take Lornit syrup with or without food.
If you use more Lornit syrup than you should
Tell your doctor if you accidentally use more than you were told.
If you forget to use Lornit syrup
If you forget to take at the right time, use it as soon as you remember, then carry on as before. Do not take a double dose to make up for a forgotten dose.
If you stop using Lornit syrup
Do not stop your treatment even if you feel better unless told to do so by your doctor. If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Rarely, side effects such as nausea and vomiting may occur. These are usually temporary and do not require stopping the medication.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top end of the home page. Website link: https://www.zuventus.com/drug-safety-reporting.
By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician.
5. How to store Lornit syrup
- Keep this medicine out of the sight and reach of children.
- Store in a cool, dry place.
- Do not use this medicine after the expiry date which is stated on the bottle and carton after EXP. The expiry date refers to the last day of that month.
- Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Lornit syrup contains:
The active substances are L-Ornithine L-Aspartate (250 mg per 5 mL) and Nicotinamide (20 mg per 5 mL).
Revised on 11/24