Revostat 20 mg Tablet
Therapy Area
Cardiology
1.0 Generic Name
Rosuvastatin Tablet IP 10mg/20mg/40mg
2.0 Qualitative and quantitative composition
Revostat 10mg
Each film-coated tablet contains:
Rosuvastatin Calcium IP equivalent to Rosuvastatin 10mg
Excipients q.s.
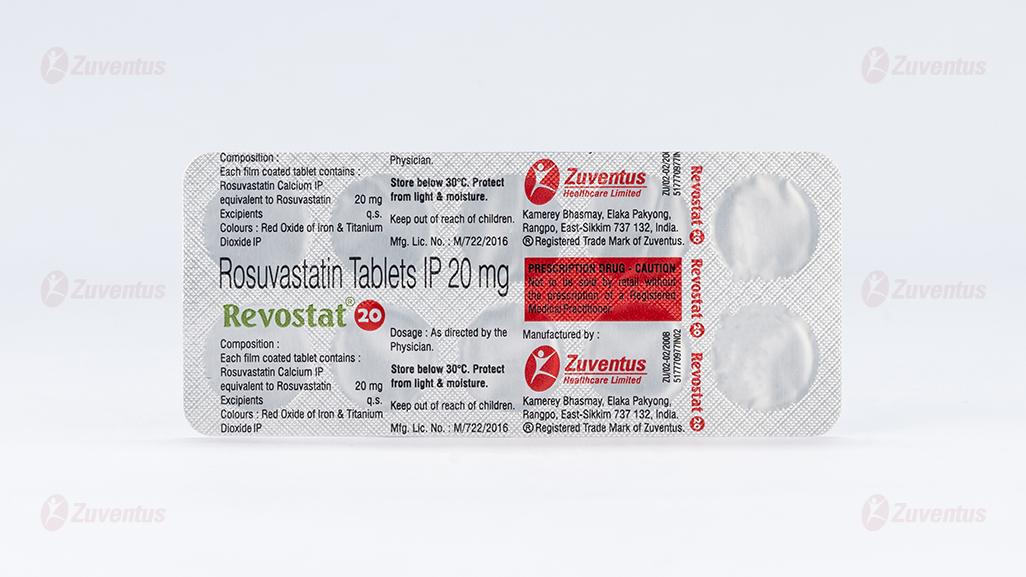
Revostat 20mg
Each film-coated tablet contains:
Rosuvastatin Calcium IP equivalent to Rosuvastatin 20mg
Excipients q.s.
Revostat 40mg
Each film-coated tablet contains:
Rosuvastatin Calcium IP equivalent to Rosuvastatin 40mg
Excipients q.s.
3.0 Dosage form and strength
Tablet 10/20/40mg
4.0 Clinical particulars
4.1 Therapeutic indication
1) As an adjunctive therapy to diet for the treatment of adult patients with hypertriglyceridemia.
2) As an adjunctive therapy to diet to slow the progression of atherosclerosis in adult patients as part of a treatment strategy to lower Total-C and LDL-C
3) Risk reduction of MI stroke and arterial evascularisation procedure in patients without clinically evident CHD but with multiple risk factor
4.2 Posology and method of administration
Before treatment initiation the patient should be placed on a standard cholesterol-lowering diet that should continue during treatment. The dose should be individualised according to the goal of therapy and patient response, using current consensus guidelines.
Revostat may be given at any time of day, with or without food.
Treatment of hypercholesterolaemia
The recommended start dose is 5 or 10 mg orally once daily in both statin naï ve or patients switched from another HMG CoA reductase inhibitor. The choice of start dose should take into account the individual patient's cholesterol level and future cardiovascular risk as well as the potential risk for adverse reactions (see below). A dose adjustment to the next dose level can be made after 4 weeks, if necessary.
Prevention of cardiovascular events
In the cardiovascular events risk reduction study, the dose used was 20 mg daily
Paediatric population
Paediatric use should only be carried out by specialists.
Children and adolescents 6 to 17 years of age (Tanner Stage< II-V)
Heterozygous familial hypercholesterolaemia
In children and adolescents with heterozygous familial hypercholesterolaemia the usual start dose is 5 mg daily.
- In children 6 to 9 years of age with heterozygous familial hypercholesterolaemia, the usual dose range is 5-10 mg orally once daily. Safety and efficacy of doses greater than 10 mg have not been studied in this population.
- In children 10 to 17 years of age with heterozygous familial hypercholesterolaemia, the usual dose range is 5-20 mg orally once daily. Safety and efficacy of doses greater than 20 mg have not been studied in this population.
Titration should be conducted according to the individual response and tolerability in paediatric patients, as recommended by the paediatric treatment recommendations (see section 4.4). Children and adolescents should be placed on standard cholesterol-lowering diet before rosuvastatin treatment initiation; this diet should be continued during rosuvastatin treatment.
Homozygous familial hypercholesterolaemia
In children 6 to 17 years of age with homozygous familial hypercholesterolaemia, the recommended maximum dose is 20 mg once daily.
A starting dose of 5 to 10 mg once daily depending on age, weight and prior statin use is advised. Titration to the maximum dose of 20 mg once daily should be conducted according to the individual response and tolerability in paediatric patients, as recommended by the paediatric treatment recommendations (see section 4.4). Children and adolescents should be placed on standard cholesterol-lowering diet before rosuvastatin treatment initiation; this diet should be continued during rosuvastatin treatment.
There is limited experience with doses other than 20 mg in this population.
The 40 mg tablet is not suitable for use in paediatric patients.
Children younger than 6 years
The safety and efficacy of use in children younger than 6 years has not been studied. Therefore, Revostat is not recommended for use in children younger than 6 years.
Use in the elderly
A start dose of 5 mg is recommended in patients>70 years. No other dose adjustment is necessary in relation to age.
Dosage in patients with renal insufficiency
No dose adjustment is necessary in patients with mild to moderate renal impairment. The recommended start dose is 5 mg in patients with moderate renal impairment (creatinine clearance <60 ml/min). The use of Revostat in patients with severe renal impairment is contraindicated for all doses.
Dosage in patients with hepatic impairment
There was no increase in systemic exposure to rosuvastatin in subjects with Child-Pugh scores of 7 or below. However, increased systemic exposure has been observed in subjects with Child-Pugh scores of 8 and 9 (see section 5.2). In these patients an assessment of renal function should be considered (see section 4.4). There is no experience in subjects with Child-Pugh scores above 9. Revostat is contraindicated in patients with active liver disease.
4.3 Contraindications
Contraindicated:
- Patients with hypersensitivity to rosuvastatin or to any of the excipients.
- Patients with active liver disease including unexplained, persistent elevations of serum transaminases and any serum transaminase elevation exceeding 3 times the upper limit of normal (ULN).
- Patients with severe renal impairment (creatinine clearance <30 ml/min).
- Patients with myopathy.
- Patients receiving concomitant combination of sofosbuvir/velpatasvir/voxilaprevir
- Patients receiving concomitant ciclosporin.
- Pregnancy and lactation and in women of childbearing potential not using appropriate contraceptive measures.
4.4 Special warnings and precautions for use
Renal Effects
Proteinuria, detected by dipstick testing and mostly tubular in origin, has been observed in patients treated with higher doses of Revostat, in particular 40 mg, where it was transient or intermittent in most cases. Proteinuria has not been shown to be predictive of acute or progressive renal disease.The reporting rate for serious renal events in post-marketing use is higher at the 40 mg dose.
Skeletal Muscle Effects
Effects on skeletal muscle e.g. myalgia, myopathy and, rarely, rhabdomyolysis have been reported in Revostat-treated patients with all doses and in particular with doses > 20 mg. Very rare cases of rhabdomyolysis have been reported with the use of ezetimibe in combination with HMG-CoA reductase inhibitors. A pharmacodynamic interaction cannot be excluded (see section 4.5) and caution should be exercised with their combined use. As with other HMG-CoA reductase inhibitors, the reporting rate for rhabdomyolysis associated with Revostat in post-marketing use is higher at the 40 mg dose.
Creatine Kinase Measurement
Creatine Kinase (CK) should not be measured following strenuous exercise or in the presence of a plausible alternative cause of CK increase which may confound interpretation of the result. If CK levels are significantly elevated at baseline (>5xULN) a confirmatory test should be carried out within 5 – 7 days. If the repeat test confirms a baseline CK >5xULN, treatment should not be started.
Before Treatment
Revostat, as with other HMG-CoA reductase inhibitors, should be prescribed with caution in patients with pre-disposing factors for myopathy/rhabdomyolysis. Such factors include:
- renal impairment
- hypothyroidism
- personal or family history of hereditary muscular disorders
- previous history of muscular toxicity with another HMG-CoA reductase inhibitor or fibrate
- alcohol abuse
- age >70 years
- situations where an increase in plasma levels may occur
- concomitant use of fibrates.
In such patients the risk of treatment should be considered in relation to possible benefit and clinical monitoring is recommended. If CK levels are significantly elevated at baseline (>5xULN) treatment should not be started.
Whilst on Treatment
Patients should be asked to report inexplicable muscle pain, weakness or cramps immediately, particularly if associated with malaise or fever. CK levels should be measured in these patients. Therapy should be discontinued if CK levels are markedly elevated (>5xULN) or if muscular symptoms are severe and cause daily discomfort (even if CK levels are ≤ 5xULN). If symptoms resolve and CK levels return to normal, then consideration should be given to re-introducing Revostat or an alternative HMG-CoA reductase inhibitor at the lowest dose with close monitoring. Routine monitoring of CK levels in asymptomatic patients is not warranted. There have been very rare reports of an immune-mediated necrotising myopathy (IMNM) during or after treatment with statins, including rosuvastatin. IMNM is clinically characterised by proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment.
In few cases, statins have been reported to induce de novo or aggravate pre-existing myasthenia gravis or ocular myasthenia (see section 4.8). Revostat should be discontinued in case of aggravation of symptoms. Recurrences when the same or a different statin was (re-) administered have been reported.
In clinical trials, there was no evidence of increased skeletal muscle effects in the small number of patients dosed with Revostat and concomitant therapy. However, an increase in the incidence of myositis and myopathy has been seen in patients receiving other HMG-CoA reductase inhibitors together with fibric acid derivatives including gemfibrozil, ciclosporin, nicotinic acid, azole antifungals, protease inhibitors and macrolide antibiotics. Gemfibrozil increases the risk of myopathy when given concomitantly with some HMG-CoA reductase inhibitors. Therefore, the combination of Revostat and gemfibrozil is not recommended. The benefit of further alterations in lipid levels by the combined use of Revostat with fibrates or niacin should be carefully weighed against the potential risks of such combinations.
Revostat must not be co-administered with systemic formulations of fusidic acid or within 7 days of stopping fusidic acid treatment. In patients where the use of systemic fusidic acid is considered essential, statin treatment should be discontinued throughout the duration of fusidic acid treatment. There have been reports of rhabdomyolysis (including some fatalities) in patients receiving fusidic acid and statins in combination .Patients should be advised to seek medical advice immediately if they experience any symptoms of muscle weakness, pain or tenderness. Statin therapy may be re-introduced seven days after the last dose of fusidic acid. In exceptional circumstances, where prolonged systemic fusidic acid is needed, e.g. for the treatment of severe infections, the need for co-administration of Revostat and fusidic acid should only be considered on a case by case basis and under close medical supervision.
Revostat should not be used in any patient with an acute, serious condition suggestive of myopathy or predisposing to the development of renal failure secondary to rhabdomyolysis (e.g. sepsis, hypotension, major surgery, trauma, severe metabolic, endocrine and electrolyte disorders; or uncontrolled seizures).
Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions including Stevens-Johnson syndrome (SJS) and drug reaction with eosinophilia and systemic symptoms (DRESS), which could be life-threatening or fatal, have been reported with rosuvastatin .At the time of prescription, patients should be advised of the signs and symptoms of severe skin reactions and be closely monitored. If signs and symptoms suggestive of this reaction appear, Revostat should be discontinued immediately and an alternative treatment should be considered.
If the patient has developed a serious reaction such as SJS or DRESS with the use of Revostat, treatment with Revostat must not be restarted in this patient at any time.
Liver Effects
As with other HMG-CoA reductase inhibitors, Revostat should be used with caution in patients who consume excessive quantities of alcohol and/or have a history of liver disease.
It is recommended that liver function tests be carried out prior to, and 3 months following, the initiation of treatment. Revostat should be discontinued or the dose reduced if the level of serum transaminases is greater than 3 times the upper limit of normal. The reporting rate for serious hepatic events (consisting mainly of increased hepatic transaminases) in post-marketing use is higher at the 40 mg dose.
In patients with secondary hypercholesterolaemia caused by hypothyroidism or nephrotic syndrome, the underlying disease should be treated prior to initiating therapy with Revostat.
Race
Pharmacokinetic studies show an increase in exposure in Asian subjects compared with Caucasians.
Protease Inhibitors
Increased systemic exposure to rosuvastatin has been observed in subjects receiving rosuvastatin concomitantly with various protease inhibitors in combination with ritonavir. Consideration should be given both to the benefit of lipid lowering by use of Revostat in HIV patients receiving protease inhibitors and the potential for increased rosuvastatin plasma concentrations when initiating and up titrating Revostat doses in patients treated with protease inhibitors. The concomitant use with certain protease inhibitors is not recommended unless the dose of Revostat is adjusted.
Lactose Intolerance
Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Interstitial Lung Disease
Exceptional cases of interstitial lung disease have been reported with some statins, especially with long-term therapy.Presenting features can include dyspnoea, non-productive cough and deterioration in general health (fatigue, weight loss and fever). If it is suspected a patient has developed interstitial lung disease, statin therapy should be discontinued.
Diabetes Mellitus
Some evidence suggests that statins as a class raise blood glucose and in some patients, at high risk of future diabetes, may produce a level of hyperglycaemia where formal diabetes care is appropriate. This risk, however, is outweighed by the reduction in vascular risk with statins and therefore should not be a reason for stopping statin treatment. Patients at risk (fasting glucose 5.6 to 6.9 mmol/l, BMI >30 kg/m2, raised triglycerides, hypertension) should be monitored both clinically and biochemically according to national guidelines.
In the JUPITER study, the reported overall frequency of diabetes mellitus was 2.8% in rosuvastatin and 2.3% in placebo, mostly in patients with fasting glucose 5.6 to 6.9 mmol/l.
Paediatric Population
The evaluation of linear growth (height), weight, BMI (body mass index), and secondary characteristics of sexual maturation by Tanner staging in paediatric patients 6 to 17 years of age taking rosuvastatin is limited to a two-year period. After two years of study treatment, no effect on growth, weight, BMI or sexual maturation was detected (see section 5.1).
In a clinical trial of children and adolescents receiving rosuvastatin for 52 weeks, CK elevations >10xULN and muscle symptoms following exercise or increased physical activity were observed more frequently compared to observations in clinical trials in adults.
4.5 Interaction with other medicinal products and other forms of interaction
Effect of co-administered medicinal products on rosuvastatin
Transporter protein inhibitors: Rosuvastatin is a substrate for certain transporter proteins including the hepatic uptake transporter OATP1B1 and efflux transporter BCRP. Concomitant administration of Revostat with medicinal products that are inhibitors of these transporter proteins may result in increased rosuvastatin plasma concentrations and an increased risk of myopathy
Ciclosporin: During concomitant treatment with Revostat and ciclosporin, rosuvastatin AUC values were on average 7 times higher than those observed in healthy volunteers (see Table 1). Revostat is contraindicated in patients receiving concomitant ciclosporin.Concomitant administration did not affect plasma concentrations of ciclosporin.
Protease inhibitors: Although the exact mechanism of interaction is unknown, concomitant protease inhibitor use may strongly increase rosuvastatin exposure. For instance, in a pharmacokinetic study, co-administration of 10 mg rosuvastatin and a combination product of two protease inhibitors (300 mg atazanavir/100 mg ritonavir) in healthy volunteers was associated with an approximately three-fold and seven-fold increase in rosuvastatin AUC and Cmax respectively. The concomitant use of Revostat and some protease inhibitor combinations may be considered after careful consideration of Revostat dose adjustments based on the expected increase in rosuvastatin exposure.
Gemfibrozil and other lipid-lowering products: Concomitant use of Revostat and gemfibrozil resulted in a 2-fold increase in rosuvastatin Cmax and AUC.
Based on data from specific interaction studies no pharmacokinetic relevant interaction with fenofibrate is expected, however a pharmacodynamic interaction may occur. Gemfibrozil, fenofibrate, other fibrates and lipid lowering doses (> or equal to 1 g/day) of niacin (nicotinic acid) increase the risk of myopathy when given concomitantly with HMG-CoA reductase inhibitors, probably because they can produce myopathy when given alone. The 40 mg dose is contraindicated with concomitant use of a fibrate (see sections 4.3 and 4.4). These patients should also start with the 5 mg dose.
Ezetimibe: Concomitant use of 10 mg Revostat and 10 mg ezetimibe resulted in a 1.2-fold increase in AUC of rosuvastatin in hypercholesterolaemic subjects.A pharmacodynamic interaction, in terms of adverse effects, between Revostat and ezetimibe cannot be ruled out.
Antacid: The simultaneous dosing of Revostat with an antacid suspension containing aluminium and magnesium hydroxide resulted in a decrease in rosuvastatin plasma concentration of approximately 50%. This effect was mitigated when the antacid was dosed 2 hours after Revostat. The clinical relevance of this interaction has not been studied.
Erythromycin: Concomitant use of Revostat and erythromycin resulted in a 20% decrease in AUC and a 30% decrease in Cmax of rosuvastatin. This interaction may be caused by the increase in gut motility caused by erythromycin.
Ticagrelor: Ticagrelor might affect renal excretion of rosuvastatin, increasing the risk for rosuvastatin accumulation. Although the exact mechanism is not known, in some cases, concomitant use of ticagrelor and rosuvastatin led to renal function decrease, increased CPK level and rhabdomyolysis.
Cytochrome P450 enzymes: Results from in vitro and in vivo studies show that rosuvastatin is neither an inhibitor nor an inducer of cytochrome P450 isoenzymes. In addition, rosuvastatin is a poor substrate for these isoenzymes. Therefore, drug interactions resulting from cytochrome P450-mediated metabolism are not expected. No clinically relevant interactions have been observed between rosuvastatin and either fluconazole (an inhibitor of CYP2C9 and CYP3A4) or ketoconazole (an inhibitor of CYP2A6 and CYP3A4).
Interactions requiring rosuvastatin dose adjustments (see also Table 1): When it is necessary to co-administer Revostat with other medicinal products known to increase exposure to rosuvastatin, doses of Revostat should be adjusted. Start with a 5 mg once daily dose of Revostat if the expected increase in exposure (AUC) is approximately 2-fold or higher. The maximum daily dose of Revostat should be adjusted so that the expected rosuvastatin exposure would not likely exceed that of a 40 mg daily dose of Revostat taken without interacting medicinal products, for example a 20 mg dose of Revostat with gemfibrozil (1.9-fold increase), and a 10 mg dose of Revostat with combination ritonavir/atazanavir (3.1-fold increase).
If medicinal product is observed to increase rosuvastatin AUC less than 2-fold, the starting dose need not be decreased but caution should be taken if increasing the Revostat dose above 20mg.
The following medical product/combinations did not have a clinically significant effect on the AUC ratio of rosuvastatin at coadministration:
Aleglitazar 0.3 mg 7 days dosing; Fenofibrate 67 mg 7 days TID dosing; Fluconazole 200mg 11 days OD dosing; Fosamprenavir 700 mg/ritonavir 100 mg 8 days BID dosing; Ketoconazole 200 mg 7 days BID dosing; Rifampin 450 mg 7 days OD dosing; Silymarin 140 mg 5 days TID dosing. Effect of rosuvastatin on co-administered medicinal products
Vitamin K antagonists: As with other HMG-CoA reductase inhibitors, the initiation of treatment or dosage up-titration of Revostat in patients treated concomitantly with vitamin K antagonists (e.g. warfarin or another coumarin anticoagulant) may result in an increase in International Normalised Ratio (INR). Discontinuation or down-titration of Revostat may result in a decrease in INR. In such situations, appropriate monitoring of INR is desirable.
Oral contraceptive/hormone replacement therapy (HRT): Concomitant use of Revostat and an oral contraceptive resulted in an increase in ethinyl estradiol and norgestrel AUC of 26% and 34%, respectively. These increased plasma levels should be considered when selecting oral contraceptive doses. There are no pharmacokinetic data available in subjects taking concomitant Revostat and HRT, therefore, a similar effect cannot be excluded. However, the combination has been extensively used in women in clinical trials and was well tolerated.
Other medicinal products:
Digoxin: Based on data from specific interaction studies no clinically relevant interaction with digoxin is expected.
Fusidic Acid: Interaction studies with rosuvastatin and fusidic acid have not been conducted. The risk of myopathy, including rhabdomyolysis may be increased by the concomitant administration of systemic fusidic acid with statins. The mechanism of this interaction (whether it is pharmacodynamic or pharmacokinetic, or both) is yet unknown. There have been reports of rhabdomyolysis (including some fatalities) in patients receiving this combination.
If treatment with systemic fusidic acid is necessary, Revostat treatment should be discontinued throughout the duration of the fusidic acid treatment. Also see section 4.4.
Paediatric population: Interaction studies have only been performed in adults. The extent of interactions in the paediatric population is not known.
4.6 Fertility, pregnancy and lactation
Revostat is contraindicated in pregnancy and lactation.
Women of child bearing potential should use appropriate contraceptive measures.
Since cholesterol and other products of cholesterol biosynthesis are essential for the development of the foetus, the potential risk from inhibition of HMG-CoA reductase outweighs the advantage of treatment during pregnancy. Animal studies provide limited evidence of reproductive toxicity . If a patient becomes pregnant during use of this product, treatment should be discontinued immediately.
Rosuvastatin is excreted in the milk of rats. There are no data with respect to excretion in milk in humans .
4.7 Effects on ability to drive and use machines
Studies to determine the effect of Revostat on the ability to drive and use machines have not been conducted. However, based on its pharmacodynamic properties, Revostat is unlikely to affect this ability. When driving vehicles or operating machines, it should be taken into account that dizziness may occur during treatment.
4.8 Undesirable effects
The adverse reactions seen with Revostat are generally mild and transient. In controlled clinical trials, less than 4% of Revostat-treated patients were withdrawn due to adverse reactions.
Tabulated list of adverse reactions Based on data from clinical studies and extensive post-marketing experience, the following table presents the adverse reaction profile for rosuvastatin. Adverse reactions listed below are classified according to frequency and system organ class (SOC).
The frequencies of adverse reactions are ranked according to the following convention: Common (≥ 1/100 to <1/10); Uncommon (≥ 1/1,000 to <1/100); Rare (≥ 1/10,000 to <1/1000); Very rare (<1/10,000); Not known (cannot be estimated from the available data).
Table 2. Adverse reactions based on data from clinical studies and post-marketing experience
As with other HMG-CoA reductase inhibitors, the incidence of adverse drug reactions tends to be dose dependent.
Renal effects: Proteinuria, detected by dipstick testing and mostly tubular in origin, has been observed in patients treated with Revostat. Shifts in urine protein from none or trace to ++ or more were seen in <1% of patients at some time during treatment with 10 and 20 mg, and in approximately 3% of patients treated with 40 mg. A minor increase in shift from none or trace to + was observed with the 20 mg dose. In most cases, proteinuria decreases or disappears spontaneously on continued therapy. Review of data from clinical trials and post-marketing experience to date has not identified a causal association between proteinuria and acute or progressive renal disease.
Haematuria has been observed in patients treated with Revostat and clinical trial data show that the occurrence is low.
Skeletal muscle effects: Effects on skeletal muscle e.g. myalgia, myopathy (including myositis) and, rarely, rhabdomyolysis with and without acute renal failure have been reported in Revostat-treated patients with all doses and in particular with doses > 20 mg.
A dose-related increase in CK levels has been observed in patients taking rosuvastatin; the majority of cases were mild, asymptomatic and transient. If CK levels are elevated (>5xULN), treatment should be discontinued.
Liver effects: As with other HMG-CoA reductase inhibitors, a dose-related increase in transaminases has been observed in a small number of patients taking rosuvastatin; the majority of cases were mild, asymptomatic and transient.
The following adverse events have been reported with some statins:
Sexual dysfunction. Exceptional cases of interstitial lung disease, especially with long term therapy .
The reporting rates for rhabdomyolysis, serious renal events and serious hepatic events (consisting mainly of increased hepatic transaminases) is higher at the 40 mg dose.
Paediatric population: Creatine kinase elevations >10xULN and muscle symptoms following exercise or increased physical activity were observed more frequently in a 52-week clinical trial of children and adolescents compared to adults .In other respects, the safety profile of rosuvastatin was similar in children and adolescents compared to adults.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
There is no specific treatment in the event of overdose. In the event of overdose, the patient should be treated symptomatically and supportive measures instituted as required. Liver function and CK levels should be monitored. Haemodialysis is unlikely to be of benefit.
5.0 Pharmacological properties
5.1 Mechanism of action
Rosuvastatin is a selective and competitive inhibitor of HMG-CoA reductase, the rate-limiting enzyme that converts 3-hydroxy-3-methylglutaryl coenzyme A to mevalonate, a precursor for cholesterol. The primary site of action of rosuvastatin is the liver, the target organ for cholesterol lowering.
Rosuvastatin increases the number of hepatic LDL receptors on the cell-surface, enhancing uptake and catabolism of LDL and it inhibits the hepatic synthesis of VLDL, thereby reducing the total number of VLDL and LDL particles.
5.2 Pharmacodynamic effects
Revostat reduces elevated LDL-cholesterol, total cholesterol and triglycerides and increases HDL-cholesterol. It also lowers ApoB, non-HDL-C, VLDL-C, VLDL-TG and increases ApoA-I (see Table 3). Revostat also lowers the LDL-C/HDL-C, total C/HDL-C and non-HDL-C/HDL-C and the ApoB/ApoA-I ratios.
Table 3 Dose response in patients with primary hypercholesterolaemia (type IIa and IIb) (adjusted mean percent change from baseline)
A therapeutic effect is obtained within 1 week following treatment initiation and 90% of maximum response is achieved in 2 weeks. The maximum response is usually achieved by 4 weeks and is maintained after that.
5.3 Pharmacokinetic properties
Absorption: Maximum rosuvastatin plasma concentrations are achieved approximately 5 hours after oral administration. The absolute bioavailability is approximately 20%.
Distribution: Rosuvastatin is taken up extensively by the liver which is the primary site of cholesterol synthesis and LDL-C clearance. The volume of distribution of rosuvastatin is approximately 134 L. Approximately 90% of rosuvastatin is bound to plasma proteins, mainly to albumin.
Metabolism: Rosuvastatin undergoes limited metabolism (approximately 10%). In vitro metabolism studies using human hepatocytes indicate that rosuvastatin is a poor substrate for cytochrome P450-based metabolism. CYP2C9 was the principal isoenzyme involved, with 2C19, 3A4 and 2D6 involved to a lesser extent. The main metabolites identified are the N-desmethyl and lactone metabolites. The N-desmethyl metabolite is approximately 50% less active than rosuvastatin whereas the lactone form is considered clinically inactive. Rosuvastatin accounts for greater than 90% of the circulating HMG-CoA reductase inhibitor activity.
Excretion: Approximately 90% of the rosuvastatin dose is excreted unchanged in the faeces (consisting of absorbed and non-absorbed active substance) and the remaining part is excreted in urine. Approximately 5% is excreted unchanged in urine. The plasma elimination half-life is approximately 19 hours. The elimination half-life does not increase at higher doses. The geometric mean plasma clearance is approximately 50 litres/hour (coefficient of variation 21.7%). As with other HMG-CoA reductase inhibitors, the hepatic uptake of rosuvastatin involves the membrane transporter OATP-C. This transporter is important in the hepatic elimination of rosuvastatin.
Linearity: Systemic exposure of rosuvastatin increases in proportion to dose. There are no changes in pharmacokinetic parameters following multiple daily doses.
6.0 Nonclinical Properties
6.1 Animal Toxicology or Pharmacology
Preclinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, genotoxicity and carcinogenicity potential. Specific tests for effects on hERG have not been evaluated. Adverse reactions not observed in clinical studies, but seen in animals at exposure levels similar to clinical exposure levels were as follows: In repeated-dose toxicity studies histopathologic liver changes likely due to the pharmacologic action of rosuvastatin were observed in mouse, rat, and to a lesser extent with effects in the gall bladder in dogs, but not in monkeys. In addition, testicular toxicity was observed in monkeys and dogs at higher dosages. Reproductive toxicity was evident in rats, with reduced litter sizes, litter weight and pup survival observed at maternally toxic doses, where systemic exposures were several times above the therapeutic exposure level.
7.0 Description
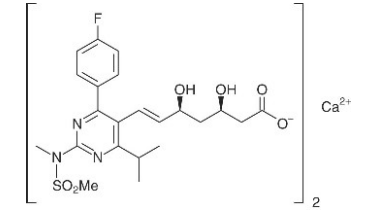
CRESTOR (rosuvastatin) is a 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA)-reductase inhibitor
Chemical Name: s bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2- [methyl(methylsulfonyl)amino] pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhept-6-enoic acid]
Molecular Formula: (C22H27FN3O6S)2Ca a
Molecular Weight: 1,001.14.
Structure:
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable.
8.2 Shelf-life
Refer on pack.
8.3 Packaging information
3 Blister strips of 10 tablets each
8.4 Storage and handing instructions
Store below 30°C. Protect from light & moisture.
Keep out of reach of children.
9.0 Patient Counselling Information
Patients should be instructed not to take 2 doses of rosuvastatin calcium within 12 hours of each other.
Skeletal Muscle Effects
Patients should be advised to report promptly unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever or if these muscle signs or symptoms persist after discontinuing rosuvastatin calcium.
Concomitant Use of Antacids
When taking rosuvastatin calcium with an aluminum and magnesium hydroxide combination antacid, the antacid should be taken at least 2 hours after rosuvastatin calcium administration.
Embryo fetal Toxicity
Advise females of reproductive potential of the risk to a fetus, to use effective contraception during treatment, and to inform their healthcare provider of a known or suspected pregnancy.
Lactation
Advise women not to breastfeed during treatment with rosuvastatin
Liver Enzymes
It is recommended that liver enzyme tests be performed before the initiation of rosuvastatin calcium and if signs or symptoms of liver injury occur. All patients treated with rosuvastatin calcium should be advised to promptly report any symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice.
12. Date of revision
10th October 2024
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side, effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet?
1) What Revostat is and what it is used for
2) What you need to know before you take Revostat
3) How to take Revostat
4) Possible side effects
5) How to store Revostat
6) Contents of the pack and other information
1. What Revostat is and what it is used for
Revostat belongs to a group of medicines called statins.
You have been prescribed Revostat because:
- You have a high cholesterol level. This means you are at risk from a heart attack or stroke. Revostat is used in adults, adolescents and children 6 years or older to treat high cholesterol.
- You have been advised to take a statin, because changing your diet and doing more exercise were not enough to correct your cholesterol levels. You should continue with your cholesterol-lowering diet and exercise while you are taking Revostat.
Or
- You have other factors that increase your risk of having a heart attack, stroke or related health problems.
Heart attack, stroke and other problems can be caused by a disease called atherosclerosis. Atherosclerosis is due to build-up of fatty deposits in your arteries.
Why it is important to keep taking Revostat
Revostat is used to correct the levels of fatty substances in the blood called lipids, the most common of which is cholesterol.
There are different types of cholesterol found in the blood – ‘bad’ cholesterol (LDL-C) and ‘good’ cholesterol (HDL-C).
- Revostat can reduce the ‘bad’ cholesterol and increase the ‘good’ cholesterol.
- It works by helping to block your body’s production of ‘bad’ cholesterol. It also improves your body’s ability to remove it from your blood.
For most people, high cholesterol does not affect the way they feel because it does not produce any symptoms. However, if it is left untreated, fatty deposits can build up in the walls of your blood vessels causing them to narrow. Sometimes, these narrowed blood vessels can get blocked which can cut off the blood supply to the heart or brain leading to a heart attack or a stroke. By lowering your cholesterol levels, you can reduce your risk of having a heart attack, a stroke or related health problems.
You need to keep taking Revostat, even if it has got your cholesterol to the right level, because it prevents your cholesterol levels from creeping up again and causing build-up of fatty deposits. However, you should stop if your doctor tells you to do so, or you have become pregnant.
2. What you need to know before you take Revostat
Do not take Revostat:
- If you have ever had an allergic reaction to Revostat, or to any of its ingredients.
- If you are pregnant or breast-feeding. If you become pregnant while taking Revostat stop taking it immediately and tell your doctor. Women should avoid becoming pregnant while taking Revostat by using suitable contraception.
- If you have liver disease.
- If you have severe kidney problems.
- If you have repeated or unexplained muscle aches or pains.
- If you take a drug combination of sofosbuvir/velpatasvir/voxilaprevir (used for viral infection of the liver called hepatitis C).
- If you take a drug called ciclosporin (used, for example, after organ transplants).
If any of the above applies to you (or you are in doubt), please go back and see your doctor.
In addition, do not take Revostat 40 mg (the highest dose):
- If you have moderate kidney problems (if in doubt, please ask your doctor).
- If your thyroid gland is not working properly.
- If you have had any repeated or unexplained muscle aches or pains, a personal or family history of muscle problems, or a previous history of muscle problems when taking other cholesterol-lowering medicines.
- If you regularly drink large amounts of alcohol.
- If you are of Asian origin (Japanese, Chinese, Filipino, Vietnamese, Korean and Indian).
- If you take other medicines called fibrates to lower your cholesterol.
If any of the above applies to you (or you are in doubt), please go back and see your doctor.
Warnings and precautions
Talk to your doctor or pharmacist before taking Revostat.
- If you have problems with your kidneys.
- If you have problems with your liver.
- If you have had repeated or unexplained muscle aches or pains, a personal or family history of muscle problems, or a previous history of muscle problems when taking other cholesterol-lowering medicines. Tell your doctor immediately if you have unexplained muscle aches or pains, especially if you feel unwell or have a fever. Also, tell your doctor or pharmacist if you have a muscle weakness that is constant.
- If you have or have had myasthenia (a disease with general muscle weakness including in some cases muscles used when breathing), or ocular myasthenia (a disease causing eye muscle weakness) as statins may sometimes aggravate the condition or lead to the occurrence of myasthenia (see section 4).
- If you have ever developed a severe skin rash or skin peeling, blistering and/or mouth sores after taking Revostat or other related medicines.
- If you regularly drink large amounts of alcohol.
- If your thyroid gland is not working properly.
- If you take other medicines called fibrates to lower your cholesterol. Please read this leaflet carefully, even if you have taken other medicines for high cholesterol before.
- If you take medicines used to treat the HIV infection e.g. ritonavir with lopinavir and/or atazanavir, please see “Other medicines and Revostat”.
- If you are taking or have taken in the last 7 days a medicine called fusidic acid (a medicine for bacterial infection), orally or by injection. The combination of fusidic acid and Revostat can lead to serious muscle problems (rhabdomyolysis), please see “Other medicines and Revostat”.
- If you are over 70 (as your doctor needs to choose the right start dose of Revostat to suit you)
- If you have severe respiratory failure.
- If you are of Asian origin – that is Japanese, Chinese, Filipino, Vietnamese, Korean and Indian. Your doctor needs to choose the right start dose of Revostat to suit you.
If any of the above applies to you (or if you are not sure):
- Do not take Revostat 40 mg (the highest dose) and check with your doctor or pharmacist before you actually start taking any dose of Revostat.
Serious skin reactions including Stevens-Johnson syndrome and drug reaction with eosinophilia and systemic symptoms (DRESS) have been reported in association with Revostat treatment. Stop using Revostat and seek medical attention immediately if you notice any of the symptoms described in section 4.
In a small number of people, statins can affect the liver. This is identified by a simple test which looks for increased levels of liver enzymes in the blood. For this reason, your doctor will usually carry out this blood test (liver function test) before and during treatment with Revostat.
While you are on this medicine your doctor will monitor you closely if you have diabetes or are at risk of developing diabetes. You are likely to be at risk of developing diabetes if you have high levels of sugars and fats in your blood, are overweight and have high blood pressure.
Children and adolescents
- If the patient is under 6 years old: Revostat should not be given to children younger than 6 years.
- If the patient is below 18 years of age: The Revostat 40 mg tablet is not suitable for use in children and adolescents below 18 years of age.
Other medicines and Revostat
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines
Tell your doctor if you are taking any of the following:
- ciclosporin (used for example, after organ transplants),
- warfarin, clopidogrel or ticagrelor (or any other drug used for thinning the blood),
- fibrates (such as gemfibrozil, fenofibrate) or any other medicine used to lower cholesterol (such as ezetimibe),
- indigestion remedies (used to neutralise acid in your stomach),
- erythromycin (an antibiotic), fusidic acid (an antibiotic – please see below and Warnings and precautions),
- an oral contraceptive (the pill),
- regorafenib (used to treat cancer),
- darolutamide (used to treat cancer),
- capmatinib (used to treat cancer),
- hormone replacement therapy,
- fostamatinib (used to treat low platelet counts),
- febuxostat (used to treat and prevent high blood levels of uric acid),
- teriflunomide (used to treat multiple sclerosis),
- any of the following drugs used to treat viral infections, including HIV or hepatitis C infection, alone or in combination (please see Warnings and precautions): ritonavir, lopinavir, atazanavir, sofosbuvir, voxilaprevir, ombitasvir, paritaprevir, dasabuvir, velpatasvir, grazoprevir, elbasvir, glecaprevir, pibrentasvir.
The effects of these medicines could be changed by Revostat or they could change the effect of Revostat.
If you need to take oral fusidic acid to treat a bacterial infection you will need to temporarily stop using this medicine. Your doctor will tell you when it is safe to restart Revostat. Taking Revostat with fusidic acid may rarely lead to muscle weakness, tenderness or pain (rhabdomyolysis). See more information regarding rhabdomyolysis.
Pregnancy and breast-feeding
Do not take Revostat if you are pregnant or breast-feeding. If you become pregnant while taking Revostat stop taking it immediately and tell your doctor. Women should avoid becoming pregnant while taking Revostat by using suitable contraception. Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Most people can drive a car and operate machinery while using Revostat – it will not affect their ability. However, some people feel dizzy during treatment with Revostat. If you feel dizzy, consult your doctor before attempting to drive or use machines.
Revostat contains lactose.
If you have been told by your doctor that you have an intolerance to some sugars (lactose or milk sugar), contact your doctor before taking Revostat.
For a full list of ingredients, please see Contents of the pack and other information.
3. How to take Revostat
Always take this medicine as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Usual doses in adults
If you are taking Revostat for high cholesterol:
Starting dose
Your treatment with Revostat must start with the 5 mg or the 10 mg dose, even if you have taken a higher dose of a different statin before. The choice of your start dose will depend upon:
- Your cholesterol levels.
- The level of risk you have of experiencing a heart attack or stroke.
- Whether you have a factor that may make you more sensitive to possible side effects.
Please check with your doctor or pharmacist which start dose of Revostat will best suit you.
Your doctor may decide to give you the lowest dose (5 mg) if:
- You are of Asian origin (Japanese, Chinese, Filipino, Vietnamese, Korean and Indian).
- You are over 70 years of age.
- You have moderate kidney problems.
- You are at risk of muscle aches and pains (myopathy).
Increasing the dose and maximum daily dose
Your doctor may decide to increase your dose. This is so that you are taking the amount of Revostat that is right for you. If you started with a 5 mg dose, your doctor may decide to double this to 10 mg, then 20 mg and then 40 mg if necessary. If you started on 10 mg, your doctor may decide to double this to 20 mg and then 40 mg if necessary. There will be a gap of four weeks between every dose adjustment.
The maximum daily dose of Revostat is 40 mg. It is only for patients with high cholesterol levels and a high risk of heart attacks or stroke whose cholesterol levels are not lowered enough with 20 mg.
If you are taking Revostat to reduce your risk of having a heart attack, stroke or related health problems:
The recommended dose is 20 mg daily. However, your doctor may decide to use a lower dose if you have any of the factors mentioned above.
Use in children and adolescents aged 6-17 years
The dose range in children and adolescents aged 6 to 17 years is 5 to 20 mg once daily. The usual start dose is 5 mg per day, and your doctor may gradually increase your dose to find the right amount of Revostat for you. The maximum daily dose of Revostat is 10 or 20 mg for children aged 6 to 17 years depending on your underlying condition being treated. Take your dose once a day. Revostat 40 mg tablet should not be used by children.
Taking your tablets
Swallow each tablet whole with a drink of water.
Take Revostat once daily. You can take it at any time of the day with or without food. Try to take your tablet at the same time every day to help you to remember it.
Regular cholesterol checks
It is important to go back to your doctor for regular cholesterol checks, to make sure your cholesterol has reached and is staying at the correct level.
Your doctor may decide to increase your dose so that you are taking the amount of Revostat that is right for you.
If you take more Revostat than you should
Contact your doctor or nearest hospital for advice.
If you go into hospital or receive treatment for another condition, tell the medical staff that you’re taking Revostat.
If you forget to take Revostat
Don’t worry, just take your next scheduled dose at the correct time. Do not take a double dose to make up for a forgotten dose.
If you stop taking Revostat
Talk to your doctor if you want to stop taking Revostat. Your cholesterol levels might increase again if you stop taking Revostat.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. It is important that you are aware of what these side effects may be. They are usually mild and disappear after a short time.
Stop taking Revostat and seek medical help immediately if you have any of the following allergic reactions:
- Difficulty in breathing, with or without swelling of the face, lips, tongue and/or throat.
- Swelling of the face, lips, tongue and/or throat, which may cause difficulty in swallowing.
- Severe itching of the skin (with raised lumps).
- Reddish non-elevated, target-like or circular patches on the trunk, often with central blisters, skin peeling, ulcers of mouth, throat, nose, genitals and eyes. These serious skin rashes can be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome).
- Widespread rash, high body temperature and enlarged lymph nodes (DRESS syndrome or drug hypersensitivity syndrome).
Also, stop taking Revostat and talk to your doctor immediately:
- If you have any unusual aches or pains in your muscles which go on for longer than you might expect. Muscle symptoms are more common in children and adolescents than in adults. As with other statins, a very small number of people have experienced unpleasant muscle effects and rarely these have gone on to become a potentially life threatening muscle damage known as rhabdomyolysis.
- If you experience muscle rupture.
- If you have lupus-like disease syndrome (including rash, joint disorders and effects on blood cells).
Common possible side effects (these may affect between 1 in 10 and 1 in 100 patients):
- Headache, stomach pain, constipation, feeling sick, muscle pain, feeling weak, dizziness.
- An increase in the amount of protein in the urine - this usually returns to normal on its own without having to stop taking your Revostat tablets (only Revostat 40 mg).
- Diabetes. This is more likely if you have high levels of sugars and fats in your blood, are overweight and have high blood pressure. Your doctor will monitor you while you are taking this medicine.
Uncommon possible side effects (these may affect between 1 in 100 and 1 in 1,000 patients):
- Rash, itching or other skin reactions.
- An increase in the amount of protein in the urine - this usually returns to normal on its own without having to stop taking your Revostat tablets (only Revostat 5 mg, 10 mg and 20 mg).
Rare possible side effects (these may affect between 1 in 1,000 and 1 in 10,000 patients):
- Severe allergic reaction – signs include swelling of the face, lips, tongue and/or throat, difficulty in swallowing and breathing, a severe itching of the skin (with raised lumps). If you think you are having an allergic reaction, then stop taking Revostat and seek medical help immediately.
- Muscle damage in adults – as a precaution, stop taking Revostat and talk to your doctor immediately if you have any unusual aches or pains in your muscles which go on for longer than expected.
- A severe stomach pain (inflamed pancreas).
- Increase in liver enzymes in the blood.
- Bleeding or bruising more easily than normal due to low level of blood platelets.
- Lupus-like disease syndrome (including rash, joint disorders and effects on blood cells).
Very rare possible side effects (these may affect less than 1 in 10,000 patients):
- Jaundice (yellowing of the skin and eyes), hepatitis (an inflamed liver), traces of blood in your urine, damage to the nerves of your legs and arms (such as numbness), joint pain, memory loss and breast enlargement in men (gynaecomastia).
Side effects of unknown frequency may include:
- Diarrhoea (loose stools), cough, shortness of breath, oedema (swelling), sleep disturbances, including insomnia and nightmares, sexual difficulties, depression, breathing problems, including persistent cough and/or shortness of breath or fever, tendon injury and muscle weakness that is constant.
- Myasthenia gravis (a disease causing general muscle weakness including in some cases muscles used when breathing), Ocular myasthenia (a disease causing eye muscle weakness). Talk to your doctor if you experience weakness in your arms or legs that worsens after periods of activity, double vision or drooping of your eyelids, difficulty swallowing, or shortness of breath.
Reporting of side effects:
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Revostat
Store below 30°C. Protect from light & moisture.
Keep out of reach of children.
6. Contents of the pack and other information
What Revostat looks like and contents of the pack
Revostat 10mg
Each film-coated tablet contains:
Rosuvastatin Calcium IP equivalent to Rosuvastatin 10mg
Excipients……………………………………………qs
Revostat 20mg
Each film-coated tablet contains:
Rosuvastatin Calcium IP equivalent to Rosuvastatin 20mg
Excipients……………………………………………qs
Revostat 40mg
Each film-coated tablet contains:
Rosuvastatin Calcium IP equivalent to Rosuvastatin 40mg
Excipients……………………………………………qs
Presentation/pack size
10 Blister strips of 10 tablets each
Marketing Authorisation Holder
Zuventus Healthcare Limited
Zuventus House, Plot Y2, CTS No.: 358/A2,
Near Nahur Railway Station,
Nahur (W), Mumbai, 400078 Maharashtra, India
This leaflet was last revised in 11/2024